MicroRNA-744-5p suppresses tumorigenesis and metastasis of osteosarcoma through the p38 mitogen-activated protein kinases pathway by targeting transforming growth factor-beta 1
- PMID: 35593122
- PMCID: PMC9276001
- DOI: 10.1080/21655979.2022.2072619
MicroRNA-744-5p suppresses tumorigenesis and metastasis of osteosarcoma through the p38 mitogen-activated protein kinases pathway by targeting transforming growth factor-beta 1
Abstract
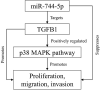
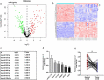
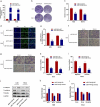
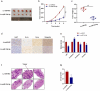
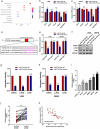
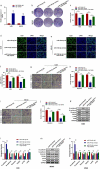
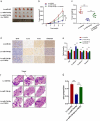
Osteosarcoma (OS) is the most common malignant bone tumor in children and adolescents. Accumulating evidence has revealed that microRNAs (miRNAs) play a crucial role in the progression of OS. In this study, we found that miR-744-5p was the least expressed miRNA in patients with OS by analyzing GSE65071 from the GENE EXPRESSION OMNIBUS (GEO) database. Through real-time quantitative PCR (qRT-PCR), western blotting, colony formation assay, 5-Ethynyl-2-Deoxyuridine (EdU) incorporation assay, transwell migration, and invasion assays, we demonstrated its ability to inhibit the proliferation, migration, and invasion of OS cells in vitro. According to the luciferase reporter assay, transforming growth factor-β1 (TGFB1) was negatively regulated by miR-744-5p and reversed the effects of miR-744-5p on OS. Subcutaneous tumor-forming animal models and tail vein injection lung metastatic models were used in animal experiments, and it was found that miR-744-5p negatively regulated tumor growth and metastasis in vivo. Furthermore, rescue assays verified that miR-744-5p regulates TGFB1 expression in OS. Further experiments revealed that the p38 MAPK signaling pathway is involved in the miR-744-5p/TGFB1 axis. Generally, this study suggests that miR-744-5p is a negative regulator of TGFB1 and suppresses OS progression and metastasis via the p38 MAPK signaling pathway.
Keywords: Osteosarcoma; TGFB1; miR-744-5p; p38 MAPK signaling pathway.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures

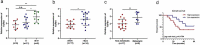
Similar articles
-
mir-744-5p inhibits cell growth and angiogenesis in osteosarcoma by targeting NFIX.J Orthop Surg Res. 2024 Aug 17;19(1):485. doi: 10.1186/s13018-024-04947-x. J Orthop Surg Res. 2024. PMID: 39152460 Free PMC article.
-
miR-624-5p promoted tumorigenesis and metastasis by suppressing hippo signaling through targeting PTPRB in osteosarcoma cells.J Exp Clin Cancer Res. 2019 Dec 11;38(1):488. doi: 10.1186/s13046-019-1491-6. J Exp Clin Cancer Res. 2019. PMID: 31829261 Free PMC article.
-
Long noncoding RNA DANCR, working as a competitive endogenous RNA, promotes ROCK1-mediated proliferation and metastasis via decoying of miR-335-5p and miR-1972 in osteosarcoma.Mol Cancer. 2018 May 12;17(1):89. doi: 10.1186/s12943-018-0837-6. Mol Cancer. 2018. PMID: 29753317 Free PMC article.
-
NEAT1 induces osteosarcoma development by modulating the miR-339-5p/TGF-β1 pathway.J Cell Physiol. 2019 Apr;234(4):5097-5105. doi: 10.1002/jcp.27313. Epub 2018 Sep 10. J Cell Physiol. 2019. PMID: 30203547
-
IncRNA MALAT1 Regulates the Proliferation, Apoptosis, Migration, and Invasion of Osteosarcoma Cells by Targeting miR-873-5p/ROCK1.Crit Rev Eukaryot Gene Expr. 2023;33(2):67-79. doi: 10.1615/CritRevEukaryotGeneExpr.2022044747. Crit Rev Eukaryot Gene Expr. 2023. PMID: 36734858
Cited by
-
mir-744-5p inhibits cell growth and angiogenesis in osteosarcoma by targeting NFIX.J Orthop Surg Res. 2024 Aug 17;19(1):485. doi: 10.1186/s13018-024-04947-x. J Orthop Surg Res. 2024. PMID: 39152460 Free PMC article.
-
Investigating novel biomarkers in uterine corpus endometrial carcinoma: in silico analysis and clinical specimens validation via RT-qPCR and immunohistochemistry.Am J Cancer Res. 2023 Sep 15;13(9):4376-4400. eCollection 2023. Am J Cancer Res. 2023. PMID: 37818076 Free PMC article.
-
Targeting nerve growth factor-mediated osteosarcoma metastasis: mechanistic insights and therapeutic opportunities using larotrectinib.Cell Death Dis. 2024 May 30;15(5):381. doi: 10.1038/s41419-024-06752-0. Cell Death Dis. 2024. PMID: 38816365 Free PMC article.
-
Integrated multiplex network based approach reveled CC and CXC chemokines associated key biomarkers in colon adenocarcinoma patients.Am J Cancer Res. 2023 Nov 15;13(11):5531-5548. eCollection 2023. Am J Cancer Res. 2023. PMID: 38058831 Free PMC article.
-
Advances in the Biological Functions and Mechanisms of miRNAs in the Development of Osteosarcoma.Technol Cancer Res Treat. 2022 Jan-Dec;21:15330338221117386. doi: 10.1177/15330338221117386. Technol Cancer Res Treat. 2022. PMID: 35950243 Free PMC article. Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
