Immunoglobulin M seroneutralization for improved confirmation of Japanese encephalitis virus infection in a flavivirus-endemic area
- PMID: 35593182
- PMCID: PMC9623734
- DOI: 10.1093/trstmh/trac036
Immunoglobulin M seroneutralization for improved confirmation of Japanese encephalitis virus infection in a flavivirus-endemic area
Abstract
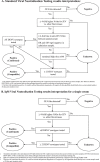
Background: The mainstay of diagnostic confirmation of acute Japanese encephalitis (JE) involves detection of anti-JE virus (JEV) immunoglobulin M (IgM) by enzyme-linked immunosorbent assay (ELISA). Limitations in the specificity of this test are increasingly apparent with the introduction of JEV vaccinations and the endemicity of other cross-reactive flaviviruses. Virus neutralization testing (VNT) is considered the gold standard, but it is challenging to implement and interpret. We performed a pilot study to assess IgG depletion prior to VNT for detection of anti-JEV IgM neutralizing antibodies (IgM-VNT) as compared with standard VNT.
Methods: We evaluated IgM-VNT in paired sera from anti-JEV IgM ELISA-positive patients (JE n=35) and negative controls of healthy flavivirus-naïve (n=10) as well as confirmed dengue (n=12) and Zika virus (n=4) patient sera. IgM-VNT was subsequently performed on single sera from additional JE patients (n=76).
Results: Anti-JEV IgG was detectable in admission serum of 58% of JE patients. The positive, negative and overall percentage agreement of IgM-VNT as compared with standard VNT was 100%. A total of 12/14 (86%) patient samples were unclassified by VNT and, with sufficient sample available for IgG depletion and IgG ELISA confirming depletion, were classified by IgM-VNT. IgM-VNT enabled JE case classification in 72/76 (95%) patients for whom only a single sample was available.
Conclusions: The novel approach has been readily adapted for high-throughput testing of single patient samples and it holds promise for incorporation into algorithms for use in reference centres.
Keywords: diagnostics, flavivirus, Laos; neglected tropical disease; neurological infection, seroneutralization.
© The Author(s) 2022. Published by Oxford University Press on behalf of Royal Society of Tropical Medicine and Hygiene.
Figures
References
-
- World Health Organization . Japanese encephalitis vaccines: WHO position paper, February 2015–recommendations. Vaccine. 2016;34(3):302–3. - PubMed
-
- Pearce JC, Learoyd TP, Langendorf BJet al. Japanese encephalitis: the vectors, ecology and potential for expansion. J Travel Med. 2018;25(Suppl 1):S16–26. - PubMed
-
- Simon-Loriere E, Faye O, Prot Met al. Autochthonous Japanese encephalitis with yellow fever coinfection in Africa. N Engl J Med. 2017;376(15):1483–5. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical