The role of cell-penetrating peptides in potential anti-cancer therapy
- PMID: 35593206
- PMCID: PMC9121317
- DOI: 10.1002/ctm2.822
The role of cell-penetrating peptides in potential anti-cancer therapy
Abstract
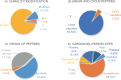
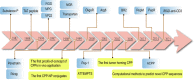
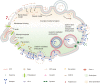
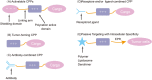
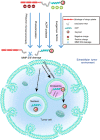
Due to the complex physiological structure, microenvironment and multiple physiological barriers, traditional anti-cancer drugs are severely restricted from reaching the tumour site. Cell-penetrating peptides (CPPs) are typically made up of 5-30 amino acids, and can be utilised as molecular transporters to facilitate the passage of therapeutic drugs across physiological barriers. Up to now, CPPs have widely been used in many anti-cancer treatment strategies, serving as an excellent potential choice for oncology treatment. However, their drawbacks, such as the lack of cell specificity, short duration of action, poor stability in vivo, compatibility problems (i.e. immunogenicity), poor therapeutic efficacy and formation of unwanted metabolites, have limited their further application in cancer treatment. The cellular uptake mechanisms of CPPs involve mainly endocytosis and direct penetration, but still remain highly controversial in academia. The CPPs-based drug delivery strategy could be improved by clever design or chemical modifications to develop the next-generation CPPs with enhanced cell penetration capability, stability and selectivity. In addition, some recent advances in targeted cell penetration that involve CPPs provide some new ideas to optimise CPPs.
Keywords: Anti-cancer therapy; cell-penetrating peptides; molecular cargoes; optimisation; tumour immunity.
© 2022 The Authors. Clinical and Translational Medicine published by John Wiley & Sons Australia, Ltd on behalf of Shanghai Institute of Clinical Bioinformatics.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
