Tumor-associated macrophages regulate the function of cytotoxic T lymphocyte through PD-1/PD-L1 pathway in multiple myeloma
- PMID: 35593325
- PMCID: PMC9761071
- DOI: 10.1002/cam4.4814
Tumor-associated macrophages regulate the function of cytotoxic T lymphocyte through PD-1/PD-L1 pathway in multiple myeloma
Abstract
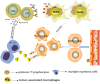
Background: Tumor-associated macrophages (TAMs) are originated from circulating mononuclear cells in peripheral blood. They result from the recruitment of tumor cells and are a vital constituent of the tumor microenvironment. TAMs may be involved in the immunological escape of vicious clonal plasma cells (PC) in the bone marrow (BM) of sufferers with myeloma.
Methods: From March 2020 to January 2021, 28 healthy controls (HC) and 86 multiple myeloma (MM) (53 newly diagnosed MM [NDMM] and 33 remissions) patients were enrolled as objects of the study. The expression of TAMs in the BM, CSF1 on CD138 + cells, and CSF1R on macrophages were detected by the method of flow cytometry, and the expression of PD-1 on CD8 + T cells and PD-L1 on TAMs were also done. Bone marrow mononuclear cells (BMMNCs) were extracted and cultured into TAMs, CD8 + T cells were sorted by magnetic beads and cultured, a coculture system was established and different inhibitors were added. The expression of the perforin and granzyme B was detected by flow cytometry.
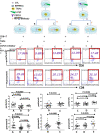
Results: The percentage of TAMs in NDMM group (61.49 ± 2.176%) increased when compared with remission (23.08 ± 1.699%, p < 0.001) and HC group (17.95 ± 1.865%, p < 0.001), and TAMs decreased after adding CSF1R inhibitor. Moreover, the expression of CSF1 on CD138 + cells increased significantly in NDMM group (17.090 ± 0.9156%) than remission (8.214 ± 0.5911% p < 0.001), and HC group (5.257 ± 0.6231%, p < 0.001), and CSF1R on macrophages increased significantly in NDMM group (58.78 ± 2.286%) than remission (20.74 ± 1.376%, p < 0.001) and HC group (17.42 ± 1.081%, p < 0.001). The expression of PD-1 on CD8 + T cells in NDMM group (32.64 ± 2.982%) increased than remission (20.35 ± 2.335% p < 0.01) and HC group (17.53 ± 1.349%, p < 0.001), and PD-L1 on TAMs also increased in NDMM group (50.92 ± 2.554%) than remission (20.02 ± 1.893%, p < 0.001) and HC group (13.08 ± 1.289%, p < 0.001). When CD8 + T cells were cocultured with TAMs, the perforin and granzyme B levels decreased significantly. However, the perforin and granzyme B levels were partly restored after adding CSF1R inhibitor and anti-PD-L1 antibody.
Conclusion: Our study shows that TAMs were increased in MM patients which can inhibit the function of cytotoxic T lymphocyte (CTL) through the PD-1/ PD-L1 signaling pathway and participate in the occurrence of immune escape of myeloma cells.
Keywords: CD8 + T cells; CSF1R; PD-1/PD-L1; TAMs; multiple myeloma.
© 2022 The Authors. Cancer Medicine published by John Wiley & Sons Ltd.
Conflict of interest statement
All authors announce that they have no conflict of interest.
Figures


Similar articles
-
Bone marrow-derived mesenchymal stem cells inhibit CD8+ T cell immune responses via PD-1/PD-L1 pathway in multiple myeloma.Clin Exp Immunol. 2021 Jul;205(1):53-62. doi: 10.1111/cei.13594. Epub 2021 May 7. Clin Exp Immunol. 2021. PMID: 33735518 Free PMC article.
-
[Expression and Significance of PD-1, PD-L1 and CTLA-4 in the Bone Marrow of Patients with Multiple Myeloma].Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2022 Dec;30(6):1803-1809. doi: 10.19746/j.cnki.issn.1009-2137.2022.06.027. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2022. PMID: 36476907 Chinese.
-
Disruption of tumour-associated macrophage trafficking by the osteopontin-induced colony-stimulating factor-1 signalling sensitises hepatocellular carcinoma to anti-PD-L1 blockade.Gut. 2019 Sep;68(9):1653-1666. doi: 10.1136/gutjnl-2019-318419. Epub 2019 Mar 22. Gut. 2019. PMID: 30902885
-
Tumor-Associated Macrophages Regulate PD-1/PD-L1 Immunosuppression.Front Immunol. 2022 May 3;13:874589. doi: 10.3389/fimmu.2022.874589. eCollection 2022. Front Immunol. 2022. PMID: 35592338 Free PMC article. Review.
-
Roles of tumor-associated macrophages in anti-PD-1/PD-L1 immunotherapy for solid cancers.Mol Cancer. 2023 Mar 21;22(1):58. doi: 10.1186/s12943-023-01725-x. Mol Cancer. 2023. PMID: 36941614 Free PMC article. Review.
Cited by
-
Advances in immunotherapy of M2 macrophages and gastrointestinal stromal tumor.World J Gastrointest Oncol. 2024 Jul 15;16(7):2915-2924. doi: 10.4251/wjgo.v16.i7.2915. World J Gastrointest Oncol. 2024. PMID: 39072184 Free PMC article. Review.
-
Tumor-Associated Macrophages in Multiple Myeloma: Key Role in Disease Biology and Potential Therapeutic Implications.Curr Oncol. 2023 Jun 25;30(7):6111-6133. doi: 10.3390/curroncol30070455. Curr Oncol. 2023. PMID: 37504315 Free PMC article. Review.
-
Macrophage depletion restores the DRG microenvironment and prevents axon degeneration in bortezomib-induced neuropathy.bioRxiv [Preprint]. 2025 Jan 24:2025.01.22.634362. doi: 10.1101/2025.01.22.634362. bioRxiv. 2025. PMID: 39896673 Free PMC article. Preprint.
-
miR-34a promotes the immunosuppressive function of multiple myeloma-associated macrophages by dampening the TLR-9 signaling.Cancer Med. 2024 Jun;13(11):e7387. doi: 10.1002/cam4.7387. Cancer Med. 2024. PMID: 38864479 Free PMC article.
-
Selinexor's Immunomodulatory Impact in Advancing Multiple Myeloma Treatment.Cells. 2025 Mar 13;14(6):430. doi: 10.3390/cells14060430. Cells. 2025. PMID: 40136679 Free PMC article. Review.
References
-
- Kumar SK, Rajkumar V, Kyle RA, et al. Multiple myeloma. Multiple Myeloma Nat Rev Dis Primers. 2017;3:17046. - PubMed
-
- Moreau P, Attal M, Facon T. Frontline therapy of multiple myeloma. Blood. 2015;125(20):3076‐3084. - PubMed
-
- Yunna C, Mengru H, Lei W, Weidong C. Macrophage M1/M2 polarization. Eur J Pharmacol. 2020;877:173090. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

