A Review of the Ring Trial Design for Evaluating Ring Interventions for Infectious Diseases
- PMID: 35593400
- PMCID: PMC10362935
- DOI: 10.1093/epirev/mxac003
A Review of the Ring Trial Design for Evaluating Ring Interventions for Infectious Diseases
Abstract
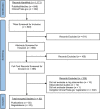
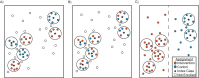
In trials of infectious disease interventions, rare outcomes and unpredictable spatiotemporal variation can introduce bias, reduce statistical power, and prevent conclusive inferences. Spillover effects can complicate inference if individual randomization is used to gain efficiency. Ring trials are a type of cluster-randomized trial that may increase efficiency and minimize bias, particularly in emergency and elimination settings with strong clustering of infection. They can be used to evaluate ring interventions, which are delivered to individuals in proximity to or contact with index cases. We conducted a systematic review of ring trials, compare them with other trial designs for evaluating ring interventions, and describe strengths and weaknesses of each design. Of 849 articles and 322 protocols screened, we identified 26 ring trials, 15 cluster-randomized trials, 5 trials that randomized households or individuals within rings, and 1 individually randomized trial. The most common interventions were postexposure prophylaxis (n = 23) and focal mass drug administration and screening and treatment (n = 7). Ring trials require robust surveillance systems and contact tracing for directly transmitted diseases. For rare diseases with strong spatiotemporal clustering, they may have higher efficiency and internal validity than cluster-randomized designs, in part because they ensure that no clusters are excluded from analysis due to zero cluster incidence. Though more research is needed to compare them with other types of trials, ring trials hold promise as a design that can increase trial speed and efficiency while reducing bias.
Keywords: disease elimination; emerging infections; postexposure prophylaxis; randomized controlled trials; reactive interventions; ring trials; ring vaccination; targeted interventions.
© The Author(s) 2022. Published by Oxford University Press on behalf of the Johns Hopkins Bloomberg School of Public Health. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Figures
Similar articles
-
The future of Cochrane Neonatal.Early Hum Dev. 2020 Nov;150:105191. doi: 10.1016/j.earlhumdev.2020.105191. Epub 2020 Sep 12. Early Hum Dev. 2020. PMID: 33036834
-
The ring vaccination trial design for the estimation of vaccine efficacy and effectiveness during infectious disease outbreaks.Clin Trials. 2022 Aug;19(4):402-406. doi: 10.1177/17407745211073594. Epub 2022 Jan 21. Clin Trials. 2022. PMID: 35057647 Free PMC article. Clinical Trial.
-
Design of trials for interrupting the transmission of endemic pathogens.Trials. 2016 Jun 6;17(1):278. doi: 10.1186/s13063-016-1378-1. Trials. 2016. PMID: 27266269 Free PMC article.
-
Cluster over individual randomization: are study design choices appropriately justified? Review of a random sample of trials.Clin Trials. 2020 Jun;17(3):253-263. doi: 10.1177/1740774519896799. Epub 2020 May 5. Clin Trials. 2020. PMID: 32367741 Review.
-
Design and analysis issues in cluster-randomized trials of interventions against infectious diseases.Stat Methods Med Res. 2000 Apr;9(2):95-116. doi: 10.1177/096228020000900203. Stat Methods Med Res. 2000. PMID: 10946429 Review.
Cited by
-
Motivations for enrollment in a COVID-19 ring-based post-exposure prophylaxis trial: qualitative examination of participant experiences.BMC Med Res Methodol. 2024 Nov 5;24(1):267. doi: 10.1186/s12874-024-02394-0. BMC Med Res Methodol. 2024. PMID: 39501157 Free PMC article.
References
-
- Foege WH, Millar JD, Lane JM. Selective epidemiologic control in smallpox eradication. Am J Epidemiol. 1971;94(4):311–315. - PubMed
-
- Corbett MS, Higgins JPT, Woolacott NF. Assessing baseline imbalance in randomised trials: implications for the Cochrane risk of bias tool. Res Synth Methods. 2014;5(1):79–85. - PubMed

