Machine Learning on a Robotic Platform for the Design of Polymer-Protein Hybrids
- PMID: 35593444
- PMCID: PMC9339531
- DOI: 10.1002/adma.202201809
Machine Learning on a Robotic Platform for the Design of Polymer-Protein Hybrids
Abstract
Polymer-protein hybrids are intriguing materials that can bolster protein stability in non-native environments, thereby enhancing their utility in diverse medicinal, commercial, and industrial applications. One stabilization strategy involves designing synthetic random copolymers with compositions attuned to the protein surface, but rational design is complicated by the vast chemical and composition space. Here, a strategy is reported to design protein-stabilizing copolymers based on active machine learning, facilitated by automated material synthesis and characterization platforms. The versatility and robustness of the approach is demonstrated by the successful identification of copolymers that preserve, or even enhance, the activity of three chemically distinct enzymes following exposure to thermal denaturing conditions. Although systematic screening results in mixed success, active learning appropriately identifies unique and effective copolymer chemistries for the stabilization of each enzyme. Overall, this work broadens the capabilities to design fit-for-purpose synthetic copolymers that promote or otherwise manipulate protein activity, with extensions toward the design of robust polymer-protein hybrid materials.
Keywords: Bayesian optimization; active learning; combinatorial polymer design; machine learning; polymer-protein conjugates; protein formulations; single-enzyme nanoparticles.
© 2022 The Authors. Advanced Materials published by Wiley-VCH GmbH.
Conflict of interest statement
Conflict of Interest
M.J.T. and A.J.G. have filed a PCT patent application and are co-founders of Plexymer, Inc.
Figures



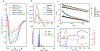
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

