VEGF-A controls the expression of its regulator of angiogenic functions, dopamine D2 receptor, on endothelial cells
- PMID: 35593650
- PMCID: PMC9234670
- DOI: 10.1242/jcs.259617
VEGF-A controls the expression of its regulator of angiogenic functions, dopamine D2 receptor, on endothelial cells
Abstract
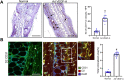
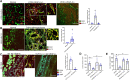
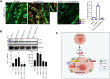
We have previously demonstrated significant upregulation of dopamine D2 (DAD2) receptor (DRD2) expression on tumor endothelial cells. The dopamine D2 receptors, upon activation, inhibit the proangiogenic actions of vascular endothelial growth factor-A (VEGF-A, also known as vascular permeability factor). Interestingly, unlike tumor endothelial cells, normal endothelial cells exhibit very low to no expression of dopamine D2 receptors. Here, for the first time, we demonstrate that through paracrine signaling, VEGF-A can control the expression of dopamine D2 receptors on endothelial cells via Krüppel-like factor 11 (KLF11)-extracellular signal-regulated kinase (ERK) 1/2 pathway. These results thus reveal a novel bidirectional communication between VEGF-A and DAD2 receptors.
Keywords: Dopamine D2 receptor; Endothelial cell; VEGF-A.
© 2022. Published by The Company of Biologists Ltd.
Conflict of interest statement
Competing interests The authors declare no competing or financial interests.
Figures

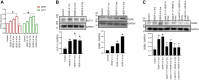
References
-
- Basu, S., Nagy, J. A., Pal, S., Vasile, E., Eckelhoefer, I. A., Bliss, V. S., Manseau, E. J., Dasgupta, P. S., Dvorak, H. F. and Mukhopadhyay, D. (2001). The neurotransmitter dopamine inhibits angiogenesis induced by vascular permeability factor/vascular endothelial growth factor. Nat. Med. 7, 569-574. 10.1038/87895 - DOI - PubMed
-
- Basu, S., Sarkar, C., Chakroborty, D., Nagy, J., Mitra, R. B., Dasgupta, P. S. and Mukhopadhyay, D. (2004). Ablation of peripheral dopaminergic nerves stimulates malignant tumor growth by inducing vascular permeability factor/vascular endothelial growth factor-mediated angiogenesis. Cancer Res. 64, 5551-5555. 10.1158/0008-5472.CAN-04-1600 - DOI - PubMed
-
- Bryant, K. L., Stalnecker, C. A., Zeitouni, D., Klomp, J. E., Peng, S., Tikunov, A. P., Gunda, V., Pierobon, M., Waters, A. M., George, S. D.et al. (2019). Combination of ERK and autophagy inhibition as a treatment approach for pancreatic cancer. Nat. Med. 25, 628-640. 10.1038/s41591-019-0368-8 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

