Phenyl Sulfones: A Route to a Diverse Family of Trisubstituted Cyclohexenes from Three Independent Nucleophilic Additions
- PMID: 35593716
- PMCID: PMC9205081
- DOI: 10.1021/jacs.2c03529
Phenyl Sulfones: A Route to a Diverse Family of Trisubstituted Cyclohexenes from Three Independent Nucleophilic Additions
Abstract
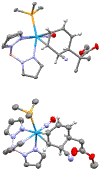
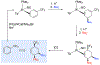
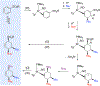
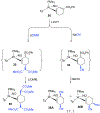
A novel process is described for the synthesis of di- and trisubstituted cyclohexenes from an arene. These compounds are prepared from three independent nucleophilic addition reactions to a phenyl sulfone (PhSO2R; R = Me, Ph, and NC4H8) dihapto-coordinated to the tungsten complex {WTp(NO)(PMe3)}(Tp = trispyrazolylborate). Such a coordination renders the dearomatized aryl ring susceptible to protonation at a carbon ortho to the sulfone group. The resulting arenium species readily reacts with the first nucleophile to form a dihapto-coordinated sulfonylated diene complex. This complex can again be protonated, and the subsequent nucleophilic addition forms a trisubstituted cyclohexene species bearing a sulfonyl group at an allylic position. Loss of the sulfinate anion forms a π-allyl species, to which a third nucleophile can be added. The trisubstituted cyclohexene can then be oxidatively decomplexed, either before or after substitution of the sulfonyl group. Nucleophiles employed include masked enolates, cyanide, amines, amides, and hydride, with all three additions occurring to the same face of the ring, anti to the metal. Of the 12 novel functionalized cyclohexenes prepared as examples of this methodology, nine compounds meet five independent criteria for evaluating drug likeliness. Structural assignments are supported with nine crystal structures, density functional theory studies, and full 2D NMR analysis.
Conflict of interest statement
The authors declare no competing financial interest.
Figures










References
-
- Tan DS, Diversity-oriented synthesis: exploring the intersections between chemistry and biology. Nat. Chem. Biol 2005, 1, 74–84. - PubMed
-
- Lovering F; Bikker J; Humblet C, Escape from Flatland: Increasing Saturation as an Approach to Improving Clinical Success. J. Med. Chem 2009, 52, 6752–6756. - PubMed
-
- Ishikawa M; Hashimoto Y, Improvement in Aqueous Solubility in Small Molecule Drug Discovery Programs by Disruption of Molecular Planarity and Symmetry. J. Med. Chem 2011, 54, 1539–1554. - PubMed
-
- Taylor RD; MacCoss M; Lawson ADG, Rings in Drugs. J. Med. Chem 2014, 57, 5845–5859. - PubMed
-
- Vitaku E; Smith DT; Njardarson JT, Analysis of the Structural Diversity, Substitution Patterns, and Frequency of Nitrogen Heterocycles among U.S. FDA Approved Pharmaceuticals. J. Med. Chem 2014, 57, 10257–10274. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

