Rapid degradation of protein tyrosine phosphatase 1B in sickle cells: Possible contribution to sickle cell membrane weakening
- PMID: 35593742
- PMCID: PMC9215175
- DOI: 10.1096/fj.202100809RR
Rapid degradation of protein tyrosine phosphatase 1B in sickle cells: Possible contribution to sickle cell membrane weakening
Abstract
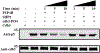
Although both protein tyrosine phosphatases and kinases are constitutively active in healthy human red blood cells (RBCs), the preponderance of phosphatase activities maintains the membrane proteins in a predominantly unphosphorylated state. We report here that unlike healthy RBCs, proteins in sickle cells are heavily tyrosine phosphorylated, raising the question regarding the mechanism underpinning this tyrosine phosphorylation. Upon investigating possible causes, we observe that protein tyrosine phosphatase 1B (PTP1B), the major erythrocyte tyrosine phosphatase, is largely digested to a lower molecular weight fragment in sickle cells. We further find that the resulting truncated form of PTP1B is significantly less active than its intact counterpart, probably accounting for the intense tyrosine phosphorylation of Band 3 in sickle erythrocytes. Because this tyrosine phosphorylation of Band 3 promotes erythrocyte membrane weakening that causes release of both membrane vesicles and cell free hemoglobin that in turn initiates vaso-occlusive events, we conclude that cleavage of PTP1B could contribute to the symptoms of sickle cell disease. We further posit that methods to inhibit proteolysis of PTP1B could mitigate symptoms of the disease.
Keywords: Band 3 tyrosine phosphorylation; PTP1B; calpain proteolysis; cleavage of PTP1B; phosphatase activity; sickle cells.
© 2022 Federation of American Societies for Experimental Biology.
Conflict of interest statement
DISCLOSURES
The authors declare no conflict of interest.
Figures


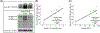
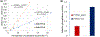

Similar articles
-
Differential sorting of tyrosine kinases and phosphotyrosine phosphatases acting on band 3 during vesiculation of human erythrocytes.Biochem J. 2004 Jan 15;377(Pt 2):489-97. doi: 10.1042/BJ20031401. Biochem J. 2004. PMID: 14527338 Free PMC article.
-
Inhibition of Band 3 tyrosine phosphorylation: a new mechanism for treatment of sickle cell disease.Br J Haematol. 2020 Aug;190(4):599-609. doi: 10.1111/bjh.16671. Epub 2020 Apr 28. Br J Haematol. 2020. PMID: 32346864 Free PMC article.
-
Activating pyruvate kinase improves red blood cell integrity by reducing band 3 tyrosine phosphorylation.Blood Adv. 2024 Nov 12;8(21):5653-5662. doi: 10.1182/bloodadvances.2024013504. Blood Adv. 2024. PMID: 39265169 Free PMC article. Clinical Trial.
-
Protein Tyrosine Phosphatase 1B (PTP1B): Insights into its New Implications in Tumorigenesis.Curr Cancer Drug Targets. 2022;22(3):181-194. doi: 10.2174/1568009622666220128113400. Curr Cancer Drug Targets. 2022. PMID: 35088671 Review.
-
Human Protein Tyrosine Phosphatase 1B (PTP1B): From Structure to Clinical Inhibitor Perspectives.Int J Mol Sci. 2022 Jun 24;23(13):7027. doi: 10.3390/ijms23137027. Int J Mol Sci. 2022. PMID: 35806030 Free PMC article. Review.
Cited by
-
Methylation profile of individuals with sickle cell trait.Epigenetics. 2025 Dec;20(1):2539234. doi: 10.1080/15592294.2025.2539234. Epub 2025 Aug 4. Epigenetics. 2025. PMID: 40758858 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

