Dressings and topical agents for the management of open wounds after surgical treatment for sacrococcygeal pilonidal sinus
- PMID: 35593897
- PMCID: PMC9121912
- DOI: 10.1002/14651858.CD013439.pub2
Dressings and topical agents for the management of open wounds after surgical treatment for sacrococcygeal pilonidal sinus
Abstract
Background: Sacrococcygeal pilonidal sinus disease is a common debilitating condition that predominantly affects young adults, with a profound impact on their activities of daily living. The condition is treated surgically, and in some cases the wound in the natal cleft is left open to heal by itself. Many dressings and topical agents are available to aid healing of these wounds.
Objectives: To assess the effects of dressings and topical agents for the management of open wounds following surgical treatment for sacrococcygeal pilonidal sinus in any care setting.
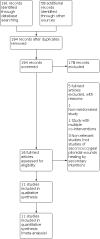
Search methods: In March 2021, we searched the Cochrane Wounds Specialised Register, CENTRAL, MEDLINE, Embase and EBSCO CINAHL Plus. We also searched clinical trials registries for ongoing and unpublished studies, and we scanned reference lists of included studies, reviews, meta-analyses and health technology reports to identify additional studies. There were no restrictions with respect to language, date of publication or study setting.
Selection criteria: We included parallel-group randomised controlled trials (RCTs) only. We included studies with participants who had undergone any type of sacrococcygeal pilonidal sinus disease surgery and were left with an open wound.
Data collection and analysis: We used the standard methodological procedures expected by Cochrane. We used GRADE to assess the certainty of the evidence for each outcome.
Main results: We included 11 RCTs comprising 932 participants. Two studies compared topical negative pressure wound therapy (TNPWT) with conventional open wound healing, two studies compared platelet-rich plasma with sterile absorbent gauze, and the other seven studies compared various dressings and topical agents. All studies were at high risk of bias in at least one domain, whilst one study was judged to be at low risk of bias in all but one domain. All studies were conducted in secondary care. Mean participant ages were between 20 and 30 years, and nearly 80% of participants were male. No studies provided data on quality of life, cost-effectiveness, pain at first dressing change or proportion of wounds healed at 6 or 12 months, and very few adverse effects were recorded in any study. It is unclear whether TNPWT reduces time to wound healing compared with conventional open wound healing (comparison 1), as the certainty of evidence is very low. The two studies provided conflicting results, with one study showing benefit (mean difference (MD) -24.01 days, 95% confidence interval (CI) -35.65 to -12.37; 19 participants), whilst the other reported no difference. It is also unclear whether TNPWT has any effect on the proportion of wounds healed by 30 days (risk ratio (RR) 3.60, 95% CI 0.49 to 26.54; 19 participants, 1 study; very low-certainty evidence). Limited data were available for our secondary outcomes time to return to normal daily activities and recurrence rate; we do not know whether TNPWT has any effect on these outcomes. Lietofix cream may increase the proportion of wounds that heal by 30 days compared with an iodine dressing (comparison 4; RR 8.06, 95% CI 1.05 to 61.68; 205 participants, 1 study; low-certainty evidence). The study did not provide data on time to wound healing. We do not know whether hydrogel dressings reduce time to wound healing compared with wound cleaning with 10% povidone iodine (comparison 5; MD -24.54 days, 95% CI -47.72 to -1.36; 31 participants, 1 study; very low-certainty evidence). The study did not provide data on the proportion of wounds healed. It is unclear whether hydrogel dressings have any effect on adverse effects as the certainty of the evidence is very low. Platelet-rich plasma may reduce time to wound healing compared with sterile absorbent gauze (comparison 6; MD -19.63 days, 95% CI -34.69 to -4.57; 210 participants, 2 studies; low-certainty evidence). No studies provided data on the proportion of wounds healed. Platelet-rich plasma may reduce time to return to normal daily activities (MD -15.49, 95% CI -28.95 to -2.02; 210 participants, 2 studies; low-certainty evidence). Zinc oxide mesh may make little or no difference to time to wound healing compared with placebo (comparison 2; median 54 days in the zinc oxide mesh group versus 62 days in the placebo mesh group; low-certainty evidence). We do not know whether zinc oxide mesh has an effect on the proportion of wounds healed by 30 days as the certainty of the evidence is very low (RR 2.35, 95% CI 0.49 to 11.23). It is unclear whether gentamicin-impregnated collagen sponge reduces time to wound healing compared with no dressing (comparison 7; MD -1.40 days, 95% CI -5.05 to 2.25; 50 participants, 1 study; very low-certainty evidence). The study did not provide data on the proportion of wounds healed. Dialkylcarbamoyl chloride (DACC)-coated dressings may make little or no difference to time to wound healing compared with alginate dressings (comparison 8; median 69 (95% CI 62 to 72) days in the DACC group versus 71 (95% CI 69 to 85) days in the alginate group; 1 study, 246 participants; low-certainty evidence). One study compared a polyurethane foam hydrophilic dressing with an alginate dressing (comparison 3) whilst another study compared a hydrocolloid dressing with an iodine dressing (comparison 9). It is unclear whether either intervention has any effect on time to wound healing as the certainty of evidence is very low.
Authors' conclusions: At present, the evidence that any of the dressings or topical agents contained in this review have a benefit on time to wound healing, the proportion of wounds that heal at a specific time point or on any of the secondary outcomes of our review ranges from low certainty to very low certainty. There is low-certainty evidence on the benefit on wound healing of platelet-rich plasma from two studies and of Lietofix cream and hydrogel dressings from single studies. Further studies are required to investigate these interventions further.
Copyright © 2022 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Conflict of interest statement
Philip J Herrod: I work as a health professional. I received a two‐year research fellowship jointly awarded by the Royal College of Surgeons of England and the Dunhill Medical Trust in 2016. I have published several articles on the management of pilonidal sinus disease previously, however none are cited in this review. I have also recently co‐authored a chapter on pilonidal sinus disease for the next edition of the Oxford Textbook of Surgery
Brett Doleman: I have received a grant from the Association of Anaesthetists of Great Britain and Ireland (AAGBI) for a randomised controlled trial of preventative paracetamol and have previously undertaken a meta‐analysis of preventative paracetamol.
Edward J Hardy: none known.
Paul Hardy: I work as a health professional. I have carried out consultancy for Smith & Nephew. The funding for this was not received by me personally, and I did not benefit from this payment or have access to the funding.
Trevor Maloney: I work as a health professional. Conference fees and travel and accommodation expenses were paid on my behalf by Convatec PLC in 2017. This funding was not paid directly to me or my host institution.
John P Williams: I work as a health professional.
Jon N Lund: I work as a health professional.
Figures












Update of
- doi: 10.1002/14651858.CD013439
Similar articles
-
Dressings and topical agents for treating pressure ulcers.Cochrane Database Syst Rev. 2017 Jun 22;6(6):CD011947. doi: 10.1002/14651858.CD011947.pub2. Cochrane Database Syst Rev. 2017. PMID: 28639707 Free PMC article.
-
Hydrogel dressings for venous leg ulcers.Cochrane Database Syst Rev. 2022 Aug 5;8(8):CD010738. doi: 10.1002/14651858.CD010738.pub2. Cochrane Database Syst Rev. 2022. PMID: 35930364 Free PMC article.
-
Topical antimicrobial agents for treating foot ulcers in people with diabetes.Cochrane Database Syst Rev. 2017 Jun 14;6(6):CD011038. doi: 10.1002/14651858.CD011038.pub2. Cochrane Database Syst Rev. 2017. PMID: 28613416 Free PMC article.
-
Foam dressings for treating pressure ulcers.Cochrane Database Syst Rev. 2017 Oct 12;10(10):CD011332. doi: 10.1002/14651858.CD011332.pub2. Cochrane Database Syst Rev. 2017. PMID: 29025198 Free PMC article.
-
Antiseptics for burns.Cochrane Database Syst Rev. 2017 Jul 12;7(7):CD011821. doi: 10.1002/14651858.CD011821.pub2. Cochrane Database Syst Rev. 2017. PMID: 28700086 Free PMC article.
Cited by
-
Efficacy and safety of RD2 Ver.02, a whole blood clot therapy, coupled with a minimally invasive procedure in pilonidal sinus: a phase II study.Tech Coloproctol. 2024 Aug 13;28(1):97. doi: 10.1007/s10151-024-02973-9. Tech Coloproctol. 2024. PMID: 39136828 Clinical Trial.
-
Pilonidal sinus disease: a brief guide for primary care.Br J Gen Pract. 2023 Dec 28;74(738):44-45. doi: 10.3399/bjgp24X736113. Print 2024 Jan. Br J Gen Pract. 2023. PMID: 38154940 Free PMC article. No abstract available.
-
What is the Impact of Negative Pressure Wound Therapy on Healing in Patients Post Excision of Pilonidal Sinus? A Systematic Review and Meta-Analysis.Int Wound J. 2025 May;22(5):e70194. doi: 10.1111/iwj.70194. Int Wound J. 2025. PMID: 40288765 Free PMC article. Review.
-
Midline and off-midline wound closure methods after surgical treatment for pilonidal sinus.Cochrane Database Syst Rev. 2024 Jan 16;1(1):CD015213. doi: 10.1002/14651858.CD015213.pub2. Cochrane Database Syst Rev. 2024. PMID: 38226663 Free PMC article.
-
Pediatric endoscopic pilonidal sinus treatment: lessons learned after 100 consecutive cases.Tech Coloproctol. 2024 Dec 10;29(1):14. doi: 10.1007/s10151-024-03049-4. Tech Coloproctol. 2024. PMID: 39656288
References
References to studies included in this review
Agren 2005 {published data only}
-
- Agren MS, Ostenfeld U, Kallehave F, Gong Y, Raffn K, Crawford ME, et al.A randomized, double-blind, placebo-controlled multicenter trial evaluating topical zinc oxide for acute open wounds following pilonidal disease excision. Wound Repair and Regeneration 2006;14:526-35. - PubMed
Banasiewicz 2013 {published data only}
-
- Banasiewicz T, Bobkiewicz A, Borejsza-Wysocki M, Biczysko M, Ratajczak A, Malinger S, et al.Portable VAC therapy improve the results of the treatment of the pilonidal sinus – randomized prospective study. Polish Journal of Surgery 2013;85:371-6. - PubMed
Berry 1996 {published data only}
-
- Berry DP, Harding KG.Dressings for treating cavity wounds. Journal of Wound care 1996;5:10-3. - PubMed
Biter 2014 {published data only}
-
- Biter LU, Beck GM, Mannaerts GH, Stok MM, Van der Ham AC, Grotenhuis BA.The use of negative-pressure wound therapy in pilonidal sinus disease: a randomized controlled trial comparing negative-pressure wound therapy versus standard open wound care after surgical excision. Diseases of the Colon and Rectum 2014;57:1406-11. - PubMed
Giannini 2019 {published data only}
-
- Giannini I, Andreoli R, Bianchi FP, Cavallaro V, Corno F, Geccherle A, et al.Effectiveness of topical use of Lietofix® in wound healing after pilonidalis sinus excision: a multicenter study by the Italian Society of Colorectal Surgery (SICCR). Techniques in Coloproctology 2019;23:373-8. - PubMed
Gohar 2020 {published data only}
Kayaoglu 2006 {published data only}
-
- Kayaoglu HA, Ozkan N, Ersoy OF, Celik A.The effect of hydrogel use on wound healing in pilonidal sinus patients undergoing surgical therapy by lay open technique [Sekonder iyileşmeye bırakılan pilonidal sinüs olgularında hidrojel kullanımının yara iyileşmesi üzerine etkisi]. Turkish Journal of Surgery 2006;22:26-9.
Mohammadi 2017 {published data only}
-
- Mohammadi M, Nasiri S, Mohammadi MH, Mohammadi AM, Nikbakht M, Panah MZ, et al.Evaluation of platelet-rich plasma gel potential in acceleration of wound healing duration in patients underwent pilonidal sinus surgery: a randomized controlled parallel clinical trial. Transfusion and Apheresis Science 2017;56:226-32. - PubMed
Ozbalci 2014 {published data only}
Romain 2020 {published data only}
Viciano 2000 {published data only}
-
- Viciano V, Castera JE, Medrano J, Aguilo J, Torro J, Botella MG, et al.Effect of hydrocolloid dressings on healing by second intention after excision of pilonidal sinus. European Journal of Surgery 2000;166:229-32. - PubMed
References to studies excluded from this review
Cherkasov 2016 {published data only}
-
- Cherkasov MF, Galashokyan KM, Startsev YM, Cherkasov DM, Melikova SG.Comparative study of treatment methods of pilonidal sinus. New Armenian Medical Journal 2016;4:61-5.
Panahi 2015 {published data only}
-
- Panahi Y, Izadi M, Sayyadi N, Rezaee R, Jonaidi-Jafari N, Beiraghdar F, et al.Comparative trial of aloe vera/olive oil combination cream versus phenytoin cream in the treatment of chronic wounds. Journal of Wound Care 2015;24:459-65. - PubMed
Rao 2010 {published data only}
-
- Rao MM, Zawislak W, Kennedy R, Gilliand R.A prospective randomised study comparing two treatment modalities for chronic pilonidal sinus with a 5-year follow-up. International Journal of Colorectal Disease 2010;25:395-400. - PubMed
Sadati 2019 {published data only}
-
- Sadati L, Froozesh R, Beyrami A, Khaneghah ZN, Elahi SA, Asl MF, et al.A comparison of three dressing methods for pilonidal sinus surgery wound healing. Advances in Skin and Wound Care 2019;32:1-5. - PubMed
Yetim 2010 {published data only}
-
- Yetim I, Ozkan OV, Dervisoglu A, Erzurumlu K, Canbolant E.Effect of gentamicin-absorbed collagen in wound healing in pilonidal sinus surgery: a prospective randomized study. Journal of International Medical Research 2010;38:1029-33. - PubMed
Additional references
Al‐Khamis 2010
Bendewald 2007
BNF 2019a
-
- British National Formulary.Alginate dressings. Available at: bnf.nice.org.uk/wound-management/alginate-dressings.html (accessed 19 February 2019).
BNF 2019b
-
- British National Formulary.Antimicrobial dressings. Available at: bnf.nice.org.uk/wound-management/antimicrobial-dressings.html (accessed 19 February 2019).
BNF 2019c
-
- British National Formulary.Foam dressings. Available at: bnf.nice.org.uk/wound-management/foam-dressings.html (accessed 19 February 2019).
BNF 2019d
-
- British National Formulary.Wound management. Available at: bnf.nice.org.uk/wound-management/ (accessed 08 July 2021).
Brown 2019
Chetter 2017
Collier 2017
-
- Collier M, Hofer P.Taking wound cleansing seriously to minimise risk. Wounds UK 2017;13(1):58-64.
Dabiri 2016
Deeks 2021
-
- Deeks JJ, Higgins JP, Altman DG (editors).Chapter 10: Analysing data and undertaking meta-analyses. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.2 (updated February 2021). Cochrane, 2021. Available from www.training.cochrane.org/handbook.
Doleman 2018
-
- Doleman B, Sutton AJ, Sherwin M, Lund JN, Williams JP.Baseline morphine consumption may explain between-study heterogeneity in meta-analyses of adjuvant analgesics and improve precision and accuracy of effect estimates. Anesthesia & Analgesia 2018;126:648-60. - PubMed
Doleman 2020
-
- Doleman B, Freeman SC, Lund JN, Williams JP, Sutton AJ.Funnel plots may show asymmetry in the absence of publication bias with continuous outcomes dependent on baseline risk: presentation of a new publication bias test. Research Synthesis Methods 2020;11:522-34. - PubMed
Donner 2002
-
- Donner A, Klar N.Issues in the meta-analysis of cluster randomized trials. Medical Statistics 2002;21(19):2971-80. - PubMed
Egger 1997
EuroQol 1990
-
- EuroQol Group.EuroQol - a new facility for the measurement of health-related quality of life. Health Policy 1990;16(3):199-208. - PubMed
Fowler 2012
-
- Fowler A.Hydrocolloids in practice. Available at: www.wounds-uk.com/resources/details/hydrocolloids-in-practice (accessed 19 February 2019).
Glanville 2019
-
- Glanville J, Dooley G, Wisniewski S, Foxlee R, Noel-Storr A.Development of a search filter to identify reports of controlled clinical trials within CINAHL Plus. Health Information and Libraries Journal 2019;36(1):73-90. - PubMed
Hahn 2005
Han 2017
Harris 2012
Higgins 2003
Higgins 2017
-
- Higgins JP, Altman DG, Sterne JA editor(s).Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.2.0 (updated June 2017). Cochrane, 2017. Available from: www.training.cochrane.org/handbook.
Higgins 2021
-
- Higgins JP, Eldridge S, Li T (editors).Chapter 23: Including variants on randomized trials. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.2 (updated February 2021). Cochrane, 2021. Available from www.training.cochrane.org/handbook.
Jones 2005
-
- Jones A, Vaughan D.Hydrogel dressings in the management of a variety of wound types: a review. Journal of Orthopaedic Nursing 2005;9:S1-11.
Lefebvre 2021
-
- Lefebvre C, Glanville J, Briscoe S, Littlewood A, Marshall C, Metzendorf M-I, et al.Chapter 4: Searching for and selecting studies. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (updated February 2021). Cochrane, 2021. Available from training.cochrane.org/handbook .
Li 2021
-
- Li T, Higgins JP, Deeks JJ (editors).Chapter 5: Collecting data. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.2 (updated February 2021). Available from training.cochrane.org/handbook.
Lund 2017
Mayo 1833
McCaughan 2018
Meher 2016
Onder 2012
Ouzzani 2016
Percival 2008
-
- Percival SL, Bowler P, Woods EJ.Assessing the effect of an antimicrobial wound dressing on biofilms. Wound Repair and Regeneration 2008;16(1):52-7. - PubMed
Price 2004
Review Manager 2020 [Computer program]
-
- Nordic Cochrane Centre, The Cochrane Collaboration Review Manager 5 (RevMan 5).Version 5.4.1. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2020.
Schultz 2017
-
- Schultz G, Bjarnsholt T, James GA, Leaper DJ, McBain AJ, Malone M, et al, Global Wound Biofilm Expert Panel.Consensus guidelines for the identification and treatment of biofilms in chronic nonhealing wounds. Wound Repair and Regeneration 2017;25(5):744-57. - PubMed
Schünemann 2013
-
- Schünemann H, Brożek J, Guyatt G, Oxman A, editor(s).Handbook for grading the quality of evidence and the strength of recommendations using the GRADE approach (updated October 2013). GRADE Working Group, 2013. Available from gdt.guidelinedevelopment.org/app/handbook/handbook.html.
Sian 2018
-
- Sian TS, Herrod PJ, Blackwell JE, Hardy EJ, Lund JN.Fibrin glue is a quick and effective treatment for primary and recurrent pilonidal sinus disease. Techniques in Coloproctology 2018;22(10):779-84. - PubMed
Stata [Computer program]
-
- Stata Statistical Software.Version 15. College Station (TX): StataCorp, 2017. Available at www.stata.com.
Stern 2004
-
- Stern PJ, Goldfarb CA.Interdigital Pilonidal Sinus. New England Journal of Medicine 2004;350:e10. - PubMed
Stewart 2012
-
- Stewart AM, Baker JD, Elliott D.The psychological wellbeing of patients following excision of a pilonidal sinus. Journal of Wound Care 2012;21(12):595-600. - PubMed
Søndenaa 1995a
-
- Søndenaa K, Andersen E, Nesvik I, Søreide JA.Patient characteristics and symptoms in chronic pilonidal sinus disease. International Journal of Colorectal Disease 1995;10(1):39-42. - PubMed
Søndenaa 1995b
-
- Søndenaa K, Nesvik I, Andersen E, Natås O, Søreide JA.Bacteriology and complications of chronic pilonidal sinus treated with excision and primary suture. International Journal of Colorectal Disease 1995;10(3):161-6. - PubMed
Tien 2018
-
- Tien T, Athem R, Arulampalam T.Outcomes of endoscopic pilonidal sinus treatment (EPSiT): a systematic review. Techniques in Coloproctology 2018;22(5):325-31. - PubMed
Tierney 2007
Venturi 2005
-
- Venturi ML, Attinger CE, Mesbahi AN, Hess CL, Graw KS.Mechanisms and clinical applications of the vacuum-assisted closure (VAC): a review. American Journal of Clinical Dermatology 2005;6(3):185-94. - PubMed
Ware 1992
-
- Ware JE Jr, Sherbourne CD.The MOS 36-item short-form health survey (SF-36). Conceptual framework and item selection. Med Care 1992;30(6):473-83. - PubMed
WebPlotDigitizer [Computer program]
-
- WebPlotDigitizer: web based tool to extract data from plots, images, and maps.Version 4.1. Austin (TX): Ankit Rohatgi, 8 January 2018. Available from: automeris.io/WebPlotDigitizer.
Winter 1962
-
- Winter G.Formation of the scab and the rate of epithelization of superficial wounds in the skin of the young domestic pig. Nature 1962;193:293-4. - PubMed
References to other published versions of this review
Herrod 2019
-
- Herrod PJ, Doleman B, Hardy EJ, Hardy P, Maloney T, WIlliams JP, et al.Dressings and topical agents for the management of open wounds after surgical treatment for sacrococcygeal pilonidal sinus. Cochrane Database of Systematic Reviews 2019, Issue 9. Art. No: CD013439. [DOI: 10.1002/14651858.CD013439] - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

