An evaluation of the long-term effectiveness of Gatekeeper™ intersphincteric implants for passive faecal incontinence
- PMID: 35593969
- PMCID: PMC9213285
- DOI: 10.1007/s10151-022-02630-z
An evaluation of the long-term effectiveness of Gatekeeper™ intersphincteric implants for passive faecal incontinence
Erratum in
-
Correction to: An evaluation of the long‑term effectiveness of Gatekeeper™ intersphincteric implants for passive faecal incontinence.Tech Coloproctol. 2022 Sep;26(9):767. doi: 10.1007/s10151-022-02655-4. Tech Coloproctol. 2022. PMID: 35731333 Free PMC article. No abstract available.
Abstract
Background: Implantation of Gatekeeper™ prostheses presents an option for the treatment of passive faecal incontinence (FI). Whilst preliminary results are encouraging, long-term data regarding its sustained benefit are limited. The aim of this study was to assess and evaluate the long-term clinical function and quality of life of patients with passive faecal incontinence who were treated with Gatekeeper™ prostheses.
Methods: This was a single centre, single surgeon retrospective study of prospectively collected clinical data in patients with FI treated between June 2012 and May 2019. Patients with passive FI with symptoms refractory to conservative treatment and endoanal ultrasonography showing intact or disrupted internal anal sphincter were included. Formal clinical and quality of life assessments were carried out using the St. Mark's Incontinence Score (SMIS) and Faecal Incontinence Quality of Life (FIQoL) questionnaires at baseline, 3 months, 6 months, 12 months and then annually. Endoanal ultrasonography was performed both before and after surgery.
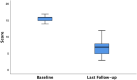
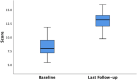
Results: Forty patients (14 males, 26 females) with a median age of 62.5 (range 33-80) years were treated with the Gatekeeper™ implant. The majority of patients (87.5%) received six implants. There were no peri or post-operative complications. Prosthesis migration was observed in 12.5% patients. The median follow-up duration was 5 years (interquartile range (IQR) 3.25-6.00 years). A sustained improvement in median SMIS and FIQoL scores from baseline to follow-up was noted. Significant differences were observed between the median baseline SMIS score and last follow-up score of 16.00 (IQR 15.00-16.75) to 7.00 (IQR 5.00-8.00) respectively (p < 0.001), a 56.25% decrease. The overall median FIQoL score showed a significant improvement from 7.95 (IQR 7.13-9.48) to 13.15 (IQR 12.00-13.98) (p < 0.001) a 65.40% increase.
Conclusions: Gatekeeper™ implantation is a safe approach to treating passive FI and is minimally invasive, reproducible and has minimal complications. Long-term sustained clinical improvement is achievable beyond 5 years. Careful patient selection is paramount, as is consistency of technique and follow-up protocol.
Keywords: Artificial anal sphincter; Bulking agents; Endoanal ultrasonography; Faecal incontinence; Gatekeeper™.
© 2022. Springer Nature Switzerland AG.
Conflict of interest statement
The authors declare that they have no conflict of interest, directly related to this study.
Figures