Trafficking regulator of GLUT4-1 (TRARG1) is a GSK3 substrate
- PMID: 35594055
- PMCID: PMC9284383
- DOI: 10.1042/BCJ20220153
Trafficking regulator of GLUT4-1 (TRARG1) is a GSK3 substrate
Abstract
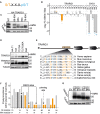
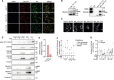
Trafficking regulator of GLUT4-1, TRARG1, positively regulates insulin-stimulated GLUT4 trafficking and insulin sensitivity. However, the mechanism(s) by which this occurs remain(s) unclear. Using biochemical and mass spectrometry analyses we found that TRARG1 is dephosphorylated in response to insulin in a PI3K/Akt-dependent manner and is a novel substrate for GSK3. Priming phosphorylation of murine TRARG1 at serine 84 allows for GSK3-directed phosphorylation at serines 72, 76 and 80. A similar pattern of phosphorylation was observed in human TRARG1, suggesting that our findings are translatable to human TRARG1. Pharmacological inhibition of GSK3 increased cell surface GLUT4 in cells stimulated with a submaximal insulin dose, and this was impaired following Trarg1 knockdown, suggesting that TRARG1 acts as a GSK3-mediated regulator in GLUT4 trafficking. These data place TRARG1 within the insulin signaling network and provide insights into how GSK3 regulates GLUT4 trafficking in adipocytes.
Keywords: adipocytes; glucose transport; glycogen synthase kinase; insulin signalling; trafficking regulator of GLUT4-1.
© 2022 The Author(s).
Conflict of interest statement
The authors declare that there are no competing interests associated with the manuscript.
Figures


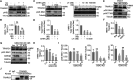
References
-
- Fazakerley, D.J., Naghiloo, S., Chaudhuri, R., Koumanov, F., Burchfield, J.G., Thomas, K.C.et al. (2015) Proteomic analysis of GLUT4 storage vesicles reveals tumor suppressor candidate 5 (TUSC5) as a novel regulator of insulin action in adipocytes. J. Biol. Chem. 290, 23528–23542 10.1074/jbc.M115.657361 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

