Development and Feasibility of a Mobile Asthma App for Children and Their Caregivers: Mixed Methods Study
- PMID: 35594073
- PMCID: PMC9166665
- DOI: 10.2196/34509
Development and Feasibility of a Mobile Asthma App for Children and Their Caregivers: Mixed Methods Study
Abstract
Background: Mobile health apps can support the self-management of pediatric asthma. Previous studies on mobile apps for children aged >7 years with asthma are limited, and most reports on asthma apps do not consider interactions between the children and their caregivers. Therefore, we developed an asthma app for children aged 0-12 years and their caregivers based on the results of our previous study regarding user needs.
Objective: The aim of this study was to evaluate the feasibility of a developed mobile app for children with asthma and their caregivers and to modify and complete the app according to the feasibility results.
Methods: We recruited children diagnosed with persistent asthma by an allergy specialist at 2 children's hospitals, 1 university hospital, 2 general hospitals, and 1 pediatric clinic. Thereafter, the app usage was assessed, and questionnaires were administered. This study used convergent mixed methods, including providing user feedback about the pediatric asthma app, completing questionnaire surveys regarding preferences, and obtaining quantitative data about app usage. Quantitative data were analyzed based on the ratings provided for the app features used by the participants, and the usage of the app features was analyzed using descriptive statistics. Qualitative data were analyzed via a descriptive qualitative research analysis and were used to identify codes from the content-characteristic words.
Results: In total, 30 pairs of children aged 2-12 years and their caregivers responded to the 3-month survey, and 20 pairs of children aged 4-12 years and their caregivers responded to the 6-month survey. In the 3- and 6-month surveys, "record" was the most commonly used feature by both caregivers and children. The average access logs per month among the 20 pairs ranged from 50 to 79 in the 6-month survey. The number of access logs decreased over time. In the qualitative results, app utilization difficulties were identified for 6 categories: record, preparing, alert settings, change settings, mobile phone owner, and display and motivation. Regarding app feasibility, 60% (12/20) of the caregivers strongly agreed or agreed for all evaluation items, while 63% (7/11) of the children strongly agreed or agreed for 6 items, excluding satisfaction. In the qualitative results, feasibility evaluation of the app was classified into 3 categories: high feasibility of the app, improvement points for the app, and personal factors preventing app utilization. Based on the results of the feasibility analysis, the final version of the app was modified and completed.
Conclusions: The app feasibility among children with asthma and their caregivers was generally good. Children aged 7-12 years used elements such as record, quiz, and manga. This app can support the continuous self-management of pediatric asthma. However, efforts must be taken to maintain and improve the app quality.
Trial registration: UMIN Clinical Trials Registry UMIN000039058; https://tinyurl.com/3na9zyf8.
Keywords: asthma; caregivers; children; feasibility; health app; mHealth; mobile app; mobile phone; pediatric; usability.
©Misa Iio, Miori Sato, Masami Narita, Kiwako Yamamoto-Hanada, Taku Oishi, Ai Kishino, Takahiro Kawaguchi, Rin Nishi, Mayumi Nagata, Yukihiro Ohya. Originally published in JMIR Formative Research (https://formative.jmir.org), 20.05.2022.
Conflict of interest statement
Conflicts of Interest: None declared.
Figures
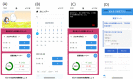
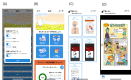
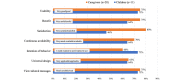
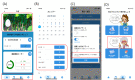
References
-
- Ferrante G, Licari A, Marseglia GL, La Grutta S. Digital health interventions in children with asthma. Clin Exp Allergy. 2021 Feb;51(2):212–220. doi: 10.1111/cea.13793. http://europepmc.org/abstract/MED/33238032 - DOI - PMC - PubMed
-
- Ramsey RR, Plevinsky JM, Kollin SR, Gibler RC, Guilbert TW, Hommel KA. Systematic Review of Digital Interventions for Pediatric Asthma Management. J Allergy Clin Immunol Pract. 2020 Apr;8(4):1284–1293. doi: 10.1016/j.jaip.2019.12.013. http://europepmc.org/abstract/MED/31870809 S2213-2198(19)31037-2 - DOI - PMC - PubMed
-
- Morrison D, Wyke S, Agur K, Cameron EJ, Docking RI, Mackenzie AM, McConnachie A, Raghuvir V, Thomson NC, Mair FS. Digital asthma self-management interventions: a systematic review. J Med Internet Res. 2014 Feb 18;16(2):e51. doi: 10.2196/jmir.2814. https://www.jmir.org/2014/2/e51/ v16i2e51 - DOI - PMC - PubMed
-
- Wu AC, Carpenter JF, Himes BE. Mobile health applications for asthma. J Allergy Clin Immunol Pract. 2015;3(3):446–8.e1. doi: 10.1016/j.jaip.2014.12.011. http://europepmc.org/abstract/MED/25725939 S2213-2198(15)00002-1 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

