Cancer Immunoediting in the Era of Immuno-oncology
- PMID: 35594163
- PMCID: PMC9481657
- DOI: 10.1158/1078-0432.CCR-21-1804
Cancer Immunoediting in the Era of Immuno-oncology
Abstract
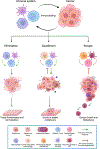
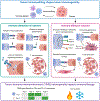
Basic science breakthroughs in T-cell biology and immune-tumor cell interactions ushered in a new era of cancer immunotherapy. Twenty years ago, cancer immunoediting was proposed as a framework to understand the dynamic process by which the immune system can both control and shape cancer and in its most complex form occurs through three phases termed elimination, equilibrium, and escape. During cancer progression through these phases, tumors undergo immunoediting, rendering them less immunogenic and more capable of establishing an immunosuppressive microenvironment. Therefore, cancer immunoediting integrates the complex immune-tumor cell interactions occurring in the tumor microenvironment and sculpts immunogenicity beyond shaping antigenicity. However, with the success of cancer immunotherapy resulting in durable clinical responses in the last decade and subsequent emergence of immuno-oncology as a clinical subspecialty, the phrase "cancer immunoediting" has recently, at times, been inappropriately restricted to describing neoantigen loss by immunoselection. This focus has obscured other mechanisms by which cancer immunoediting modifies tumor immunogenicity. Although establishment of the concept of cancer immunoediting and definitive experimental evidence supporting its existence was initially obtained from preclinical models in the absence of immunotherapy, cancer immunoediting is a continual process that also occurs during immunotherapy in human patients with cancer. Herein, we discuss the known mechanisms of cancer immunoediting obtained from preclinical and clinical data with an emphasis on how a greater understanding of cancer immunoediting may provide insights into immunotherapy resistance and how this resistance can be overcome.
©2022 American Association for Cancer Research.
Conflict of interest statement
Figures
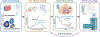


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

