Chemoselective Oxyfunctionalization of Functionalized Benzylic Compounds with a Manganese Catalyst
- PMID: 35594169
- PMCID: PMC9400980
- DOI: 10.1002/anie.202205983
Chemoselective Oxyfunctionalization of Functionalized Benzylic Compounds with a Manganese Catalyst
Abstract
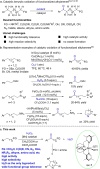
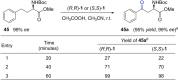
Whilst allowing for easy access to synthetically versatile motifs and for modification of bioactive molecules, the chemoselective benzylic oxidation reactions of functionalized alkyl arenes remain challenging. Reported in this study is a new non-heme Mn catalyst stabilized by a bipiperidine-based tetradentate ligand, which enables methylene oxidation of benzylic compounds by H2 O2 , showing high activity and excellent chemoselectivity under mild conditions. The protocol tolerates an unprecedentedly wide range of functional groups, including carboxylic acid and derivatives, ketone, cyano, azide, acetate, sulfonate, alkyne, amino acid, and amine units, thus providing a low-cost, more sustainable and robust pathway for the facile synthesis of ketones, increase of complexity of organic molecules, and late-stage modification of drugs.
Keywords: Benzylic Oxidation; Cyclic Imines; Ketones; Manganese Catalysts; Selective Oxidation.
© 2022 The Authors. Angewandte Chemie International Edition published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





Similar articles
-
Manganese Alkyl Carbonyl Complexes: From Iconic Stoichiometric Textbook Reactions to Catalytic Applications.Acc Chem Res. 2022 Sep 20;55(18):2740-2751. doi: 10.1021/acs.accounts.2c00470. Epub 2022 Sep 8. Acc Chem Res. 2022. PMID: 36074912 Free PMC article. Review.
-
Manganese-catalyzed selective oxidation of aliphatic C-H groups and secondary alcohols to ketones with hydrogen peroxide.ChemSusChem. 2013 Sep;6(9):1774-8. doi: 10.1002/cssc.201300378. Epub 2013 Sep 5. ChemSusChem. 2013. PMID: 24009102
-
Bioinspired Manganese and Iron Complexes for Enantioselective Oxidation Reactions: Ligand Design, Catalytic Activity, and Beyond.Acc Chem Res. 2019 Aug 20;52(8):2370-2381. doi: 10.1021/acs.accounts.9b00285. Epub 2019 Jul 23. Acc Chem Res. 2019. PMID: 31333021
-
Highly efficient oxidation of secondary alcohols to ketones catalyzed by manganese complexes of N4 ligands with H2O2.Org Lett. 2015 Jan 2;17(1):54-7. doi: 10.1021/ol5032156. Epub 2014 Dec 16. Org Lett. 2015. PMID: 25513725
-
Alkynes as Synthetic Equivalents of Ketones and Aldehydes: A Hidden Entry into Carbonyl Chemistry.Molecules. 2019 Mar 15;24(6):1036. doi: 10.3390/molecules24061036. Molecules. 2019. PMID: 30875972 Free PMC article. Review.
Cited by
-
Late-stage modification of bioactive compounds: Improving druggability through efficient molecular editing.Acta Pharm Sin B. 2024 Mar;14(3):1030-1076. doi: 10.1016/j.apsb.2023.11.021. Epub 2023 Nov 18. Acta Pharm Sin B. 2024. PMID: 38487004 Free PMC article. Review.
-
Synthesis of Δ1-Pyrrolines via Formal (3 + 2)-Cycloaddition of 2H-Azirines with Enones Promoted by Visible Light under Continuous Flow.ACS Omega. 2025 Apr 23;10(17):18017-18028. doi: 10.1021/acsomega.5c01416. eCollection 2025 May 6. ACS Omega. 2025. PMID: 40352483 Free PMC article.
-
Copper-Catalyzed Benzylic C-H Cross-Coupling Enabled by Redox Buffers: Expanding Synthetic Access to Three-Dimensional Chemical Space.Acc Chem Res. 2023 Dec 19;56(24):3604-3615. doi: 10.1021/acs.accounts.3c00580. Epub 2023 Dec 5. Acc Chem Res. 2023. PMID: 38051914 Free PMC article.
-
Chemoselective Decarboxylative Oxygenation of Carboxylic Acids To Access Ketones, Aldehydes, and Peroxides.Org Lett. 2023 Apr 14;25(14):2482-2486. doi: 10.1021/acs.orglett.3c00649. Epub 2023 Apr 4. Org Lett. 2023. PMID: 37013983 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

