Plasma membrane protrusions mediate host cell-cell fusion induced by Burkholderia thailandensis
- PMID: 35594178
- PMCID: PMC9635284
- DOI: 10.1091/mbc.E22-02-0056
Plasma membrane protrusions mediate host cell-cell fusion induced by Burkholderia thailandensis
Abstract
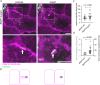
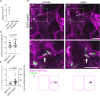
Cell-cell fusion is important for biological processes including fertilization, development, immunity, and microbial pathogenesis. Bacteria in the pseudomallei group of the Burkholderia species, including B. thailandensis, spread between host cells by inducing cell-cell fusion. Previous work showed that B. thailandensis-induced cell-cell fusion requires intracellular bacterial motility and a bacterial protein secretion apparatus called the type VI secretion system-5 (T6SS-5), including the T6SS-5 protein VgrG5. However, the cellular-level mechanism of and T6SS-5 proteins important for bacteria-induced cell-cell fusion remained incompletely described. Using live-cell imaging, we found bacteria used actin-based motility to push on the host cell plasma membrane to form plasma membrane protrusions that extended into neighboring cells. Then, membrane fusion occurred within membrane protrusions either proximal to the bacterium at the tip or elsewhere within protrusions. Expression of VgrG5 by bacteria within membrane protrusions was required to promote cell-cell fusion. Furthermore, a second predicted T6SS-5 protein, TagD5, was also required for cell-cell fusion. In the absence of VgrG5 or TagD5, bacteria in plasma membrane protrusions were engulfed into neighboring cells. Our results suggest that the T6SS-5 effectors VgrG5 and TagD5 are secreted within membrane protrusions and act locally to promote membrane fusion.
Figures




Similar articles
-
Burkholderia thailandensis uses a type VI secretion system to lyse protrusions without triggering host cell responses.Cell Host Microbe. 2024 May 8;32(5):676-692.e5. doi: 10.1016/j.chom.2024.03.013. Epub 2024 Apr 18. Cell Host Microbe. 2024. PMID: 38640929
-
The Type VI secretion system spike protein VgrG5 mediates membrane fusion during intercellular spread by pseudomallei group Burkholderia species.Infect Immun. 2014 Apr;82(4):1436-44. doi: 10.1128/IAI.01367-13. Epub 2014 Jan 13. Infect Immun. 2014. PMID: 24421040 Free PMC article.
-
Cholesterol and host cell surface proteins contribute to cell-cell fusion induced by the Burkholderia type VI secretion system 5.PLoS One. 2017 Oct 3;12(10):e0185715. doi: 10.1371/journal.pone.0185715. eCollection 2017. PLoS One. 2017. PMID: 28973030 Free PMC article.
-
The Burkholderia Type VI Secretion System 5: Composition, Regulation and Role in Virulence.Front Microbiol. 2019 Jan 10;9:3339. doi: 10.3389/fmicb.2018.03339. eCollection 2018. Front Microbiol. 2019. PMID: 30687298 Free PMC article. Review.
-
A novel contact-independent T6SS that maintains redox homeostasis via Zn2+ and Mn2+ acquisition is conserved in the Burkholderia pseudomallei complex.Microbiol Res. 2019 Sep;226:48-54. doi: 10.1016/j.micres.2019.05.007. Epub 2019 May 31. Microbiol Res. 2019. PMID: 31284944 Review.
Cited by
-
Burkholderia pseudomallei Complex Subunit and Glycoconjugate Vaccines and Their Potential to Elicit Cross-Protection to Burkholderia cepacia Complex.Vaccines (Basel). 2024 Mar 15;12(3):313. doi: 10.3390/vaccines12030313. Vaccines (Basel). 2024. PMID: 38543947 Free PMC article. Review.
-
Cryo-electron tomography of stationary phase Burkholderia thailandensis.MicroPubl Biol. 2024 Apr 24;2024:10.17912/micropub.biology.001178. doi: 10.17912/micropub.biology.001178. eCollection 2024. MicroPubl Biol. 2024. PMID: 38725941 Free PMC article.
-
The Rickettsia actin-based motility effectors RickA and Sca2 contribute differently to cell-to-cell spread and pathogenicity.mBio. 2025 Feb 5;16(2):e0256324. doi: 10.1128/mbio.02563-24. Epub 2025 Jan 17. mBio. 2025. PMID: 39819005 Free PMC article.
-
Manipulation of host cell plasma membranes by intracellular bacterial pathogens.Curr Opin Microbiol. 2023 Feb;71:102241. doi: 10.1016/j.mib.2022.102241. Epub 2022 Nov 25. Curr Opin Microbiol. 2023. PMID: 36442349 Free PMC article. Review.
-
Symbiotic T6SS affects horizontal transmission of Paraburkholderia bonniea among Dictyostelium discoideum amoeba hosts.ISME Commun. 2025 Jan 14;5(1):ycaf005. doi: 10.1093/ismeco/ycaf005. eCollection 2025 Jan. ISME Commun. 2025. PMID: 40046898 Free PMC article.
References
-
- Bulterys PL, Toesca IJ, Norris MH, Maloy JP, Fitz-Gibbon ST, France B, Toffig B, Morselli M, Somprasong N, Pellegrini M, et al. (2019). An in situ high-throughput screen identifies inhibitors of intracellular Burkholderia pseudomallei with therapeutic efficacy. Proc Natl Acad Sci USA 116, 18597–18606. - PMC - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources

