Concise Synthesis of Tunicamycin V and Discovery of a Cytostatic DPAGT1 Inhibitor
- PMID: 35594368
- PMCID: PMC9329268
- DOI: 10.1002/anie.202203225
Concise Synthesis of Tunicamycin V and Discovery of a Cytostatic DPAGT1 Inhibitor
Abstract
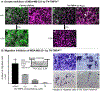
A short total synthesis of tunicamycin V (1), a non-selective phosphotransferase inhibitor, is achieved via a Büchner-Curtius-Schlotterbeck type reaction. Tunicamycin V can be synthesized in 15 chemical steps from D-galactal with 21 % overall yield. The established synthetic scheme is operationally very simple and flexible to introduce building blocks of interest. The inhibitory activity of one of the designed analogues 28 against human dolichyl-phosphate N-acetylglucosaminephosphotransferase 1 (DPAGT1) is 12.5 times greater than 1. While tunicamycins are cytotoxic molecules with a low selectivity, the novel analogue 28 displays selective cytostatic activity against breast cancer cell lines including a triple-negative breast cancer.
Keywords: Antimigration Effect; Cytostatic Activity; DPAGT1 Inhibitors; Total Synthesis; Tunicamycins.
© 2022 Wiley-VCH GmbH.
Figures





References
-
- Takatsuki A, Arima K, Tamura G, J. Antibiot 1971, 24, 215–223; - PubMed
- Takatsuki A, Tamura G, J. Antibiot 1971, 24, 224–231; - PubMed
- Takatsuki A, Tamura G, J. Antibiot 1971, 24, 232–238; - PubMed
- Takatsuki A, Tamura G, J. Antibiot 1971, 24, 785–794; - PubMed
- Ito T, Takatsuki A, Kawamura K, Sato K, Tamura G, Agric. Biol. Chem 1980, 44, 695–698;
- Kamogashira T, Takegawa S, Sugiura K, Agric. Biol. Chem 1988, 52, 859–861.
-
- Huang S, Wang D, Zhang S, Huang X, Wang D, Ijaz M, Shi Y, Cancer Chemother. Pharmacol 2017, 80, 685–696; - PubMed
- Banerjee A, Martinez JA, Longas MO, Zhang Z, Santiago J, Baksi K, Banerjee DK, Adv. Exp. Med. Biol 2015, 842, 355–374; - PMC - PubMed
- Nami B, Donmez H, Kocak N, Exp. Toxicol. Pathol 2016, 68, 419–426; - PubMed
- Hou H, Sun H, Lu P, Ge C, Zhang L, Li H, Zhao F, Tian H, Zhang L, Chen T, Yao M, Li J, Mol. Cancer Ther 2013, 12, 2874–2884; - PubMed
- Price NP, Hartman TM, Li J, Velpula KK, Naumann TA, Guda MR, Yu B, Bischoff KM, J. Antibiot 2017, 70, 1070–1077; - PubMed
- Morin MJ, Bernacki RJ, Cancer Res 1983, 43, 1669–1674; - PubMed
- Lim EJ, Heo J, Kim YH, Apoptosis 2015, 20, 1087–1098. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

