Collagen and elastic fiber remodeling in the pregnant mouse myometrium†
- PMID: 35594450
- PMCID: PMC9767674
- DOI: 10.1093/biolre/ioac102
Collagen and elastic fiber remodeling in the pregnant mouse myometrium†
Abstract
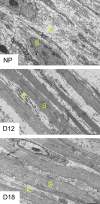
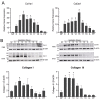
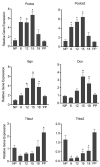
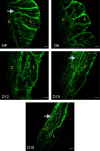
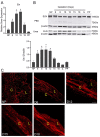
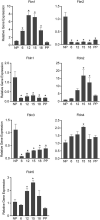
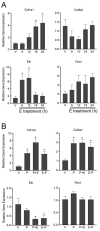
The myometrium undergoes progressive tissue remodeling from early to late pregnancy to support fetal growth and transitions to the contractile phase to deliver a baby at term. Much of our effort has been focused on understanding the functional role of myometrial smooth muscle cells, but the role of extracellular matrix is not clear. This study was aimed to demonstrate the expression profile of sub-sets of genes involved in the synthesis, processing, and assembly of collagen and elastic fibers, their structural remodeling during pregnancy, and hormonal regulation. Myometrial tissues were isolated from non-pregnant and pregnant mice to analyze gene expression and protein levels of components of collagen and elastic fibers. Second harmonic generation imaging was used to examine the morphology of collagen and elastic fibers. Gene and protein expressions of collagen and elastin were induced very early in pregnancy. Further, the gene expressions of some of the factors involved in the synthesis, processing, and assembly of collagen and elastic fibers were differentially expressed in the pregnant mouse myometrium. Our imaging analysis demonstrated that the collagen and elastic fibers undergo structural reorganization from early to late pregnancy. Collagen and elastin were differentially induced in response to estrogen and progesterone in the myometrium of ovariectomized mice. Collagen was induced by both estrogen and progesterone. By contrast, estrogen induced elastin, but progesterone suppressed its expression. The current study suggests progressive extracellular matrix remodeling and its potential role in the myometrial tissue mechanical function during pregnancy and parturition.
Keywords: collagen; elastic fibers; extracellular matrix; myometrium; steroid hormones.
© The Author(s) 2022. Published by Oxford University Press on behalf of Society for the Study of Reproduction. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Figures

Similar articles
-
Steroid Hormones Are Key Modulators of Tissue Mechanical Function via Regulation of Collagen and Elastic Fibers.Endocrinology. 2017 Apr 1;158(4):950-962. doi: 10.1210/en.2016-1930. Endocrinology. 2017. PMID: 28204185 Free PMC article.
-
Impact of Cyclic Strain on Elastin Synthesis in a 3D Human Myometrial Culture Model.Tissue Eng Part C Methods. 2024 Jul;30(7):279-288. doi: 10.1089/ten.TEC.2024.0038. Epub 2024 Jul 9. Tissue Eng Part C Methods. 2024. PMID: 38943281
-
Progesterone and gravidity differentially regulate expression of extracellular matrix components in the pregnant rat myometrium.Biol Reprod. 2004 Apr;70(4):986-92. doi: 10.1095/biolreprod.103.023648. Epub 2003 Nov 26. Biol Reprod. 2004. PMID: 14645109
-
Integration of endocrine and mechanical signals in the regulation of myometrial functions during pregnancy and labour.Eur J Obstet Gynecol Reprod Biol. 2009 May;144 Suppl 1:S2-10. doi: 10.1016/j.ejogrb.2009.02.044. Epub 2009 Mar 18. Eur J Obstet Gynecol Reprod Biol. 2009. PMID: 19299064 Review.
-
Elastic tissue disruption is a major pathogenic factor to human vascular disease.Mol Biol Rep. 2021 May;48(5):4865-4878. doi: 10.1007/s11033-021-06478-8. Epub 2021 Jun 15. Mol Biol Rep. 2021. PMID: 34129188 Review.
Cited by
-
Upregulated TIMP1 facilitates and coordinates myometrial contraction by decreasing collagens and cell adhesive capacity during human labor.Mol Hum Reprod. 2023 Sep 30;29(10):gaad034. doi: 10.1093/molehr/gaad034. Mol Hum Reprod. 2023. PMID: 37774003 Free PMC article.
-
Differential Remodelling of Endometrial Extracellular Matrix in the Non-Pregnant Uterus of Lagostomus maximus as a Potential Mechanism Underlying Embryonic Death.Animals (Basel). 2025 Feb 13;15(4):542. doi: 10.3390/ani15040542. Animals (Basel). 2025. PMID: 40003024 Free PMC article.
-
Extracellular Matrix Reorganization During Endometrial Decidualization.bioRxiv [Preprint]. 2025 Mar 25:2025.03.22.644728. doi: 10.1101/2025.03.22.644728. bioRxiv. 2025. PMID: 40196626 Free PMC article. Preprint.
-
Biomechanical and Compositional Changes in the Murine Uterus with Age.Ann Biomed Eng. 2025 Jun;53(6):1385-1398. doi: 10.1007/s10439-025-03709-y. Epub 2025 Mar 24. Ann Biomed Eng. 2025. PMID: 40126853
-
Vascular endothelial growth factor: A novel marker for torsion-induced incomplete cervical dilatation in Egyptian buffaloes.Open Vet J. 2022 Sep-Oct;12(5):709-717. doi: 10.5455/OVJ.2022.v12.i5.16. Epub 2022 Sep 19. Open Vet J. 2022. PMID: 36589390 Free PMC article.
References
-
- Wagenseil JE, Mecham RP. New insights into elastic fiber assembly. Birth Defects Res C Embryo Today 2007; 81:229–240. - PubMed

