The Eczema Area and Severity Index-A Practical Guide
- PMID: 35594457
- PMCID: PMC9154300
- DOI: 10.1097/DER.0000000000000895
The Eczema Area and Severity Index-A Practical Guide
Abstract
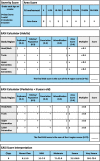
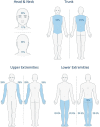
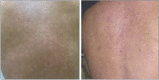
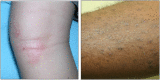
Atopic dermatitis is a chronic inflammatory skin condition that affects approximately 18 million people in the United States. Assessing the extent and severity of atopic dermatitis is critical for determining baseline disease burden and treatment effectiveness for both investigators and clinicians. Considerable efforts over the past several decades have been made in developing a highly validated instrument called the Eczema Area and Severity Index (EASI). Although several guides exist for the EASI, questions continue to arise regarding its use and interpretation. This review was developed to serve as the definitive guide for the EASI and to address commonly asked questions.
Copyright © 2022 The Author(s). Published by Wolters Kluwer Health, Inc. on behalf of the American Contact Dermatitis Society.
Conflict of interest statement
E.L.S. reports personal fees from AbbVie, Amgen, Arena Pharmaceuticals, Aslan Pharma, Boston Consulting Group, Collective Acumen, LLC (CA), Dermira, Eli Lilly, Evidera, ExcerptaMedica, Forte Bio RX, Galderma, GlaxoSmithKline, Incyte, Janssen, Kyowa Kirin Pharmaceutical Development, Leo Pharm, Medscape LLC, Pfizer, Physicians World LLC, Regeneron, Sanofi-Genzyme, Trevi Therapeutics, and WebMD. He also reports grants (or principal investigator role) from AbbVie, Amgen, Arcutis, Aslan, Corevita, Dermira, Eli Lilly, Incyte, Kyowa Hakko Kirin, Leo Pharmaceuticals, Merck, Novartis, Pfizer, Regeneron, Sanofi, and TARGET-DERM. These potential conflicts of interest have been reviewed and managed by OHSU. The other authors have no funding or conflicts of interest to declare.
Figures

References
-
- Tofte S Graeber M Cherill R, et al. . Eczema Area and Severity Index (EASI): a new tool to evaluate atopic dermatitis. J Eur Acad Dermatol Venereol 1998;11. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
