Detection of silent infection of severe acute respiratory syndrome coronavirus 2 by serological tests
- PMID: 35594509
- PMCID: PMC9122508
- DOI: 10.1371/journal.pone.0267566
Detection of silent infection of severe acute respiratory syndrome coronavirus 2 by serological tests
Abstract
Background: To control COVID-19 pandemic is of critical importance to the global public health. To capture the prevalence in an accurate and timely manner and to understand the mode of nosocomial infection are essential for its preventive measure.
Methods: We recruited 685 healthcare workers (HCW's) at Tokyo Shinagawa Hospital prior to the vaccination with COVID-19 vaccine. Sera of the subjects were tested by assays for the titer of IgG against S protein's receptor binding domain (IgG (RBD)) or IgG against nucleocapsid protein (IgG (N)) of SARS-CoV-2. Together with PCR data, the positive rates by these methods were evaluated.
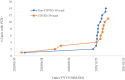
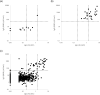
Results: Overall positive rates among HCW's by PCR, IgG (RBD), IgG (N) with a cut-off of 1.4 S/C (IgG (N)1.4), and IgG (N) with a cut-off of 0.2 S/C (IgG (N)0.2) were 3.5%, 9.5%, 6.1%, and 27.7%, respectively. Positive rates of HCW's working in COVID-19 ward were significantly higher than those of HCW's working in non-COVID-19 ward by all the four methods. Concordances of IgG (RBD), IgG (N)1.4, and IgG (N)0.2 against PCR were 97.1%, 71.4%, and 88.6%, respectively. By subtracting the positive rates of PCR from that of IgG (RBD), the rate of overall silent infection and that of HCW's in COVID-19 ward were estimated to be 6.0% and 21.1%, respectively.
Conclusions: For the prevention of nosocomial infection of SARS-CoV-2, identification of silent infection is essential. For the detection of ongoing infection, periodical screening with IgG (RBD) in addition to PCR would be an effective measure. For the surveillance of morbidity in the population, on the other hand, IgG (N)0.2 could be the most reliable indicator among the three serological tests.
Conflict of interest statement
S.S. is an employee of Abbott Japan’s. None of the other authors have competing interests. This does not alter our adherence to PLOS ONE policies on sharing data and materials.
Figures
Similar articles
-
Heterogeneous antibodies against SARS-CoV-2 spike receptor binding domain and nucleocapsid with implications for COVID-19 immunity.JCI Insight. 2020 Sep 17;5(18):e142386. doi: 10.1172/jci.insight.142386. JCI Insight. 2020. PMID: 32796155 Free PMC article.
-
Possibility of underestimation of COVID-19 prevalence by PCR and serological tests.J Microbiol Immunol Infect. 2022 Dec;55(6 Pt 1):1076-1083. doi: 10.1016/j.jmii.2021.09.005. Epub 2021 Sep 29. J Microbiol Immunol Infect. 2022. PMID: 34642099 Free PMC article.
-
Quantitative SARS-CoV-2 Serology in Children With Multisystem Inflammatory Syndrome (MIS-C).Pediatrics. 2020 Dec;146(6):e2020018242. doi: 10.1542/peds.2020-018242. Epub 2020 Sep 2. Pediatrics. 2020. PMID: 32879033
-
Serological antibody testing in the COVID-19 pandemic: their molecular basis and applications.Biochem Soc Trans. 2020 Dec 18;48(6):2851-2863. doi: 10.1042/BST20200744. Biochem Soc Trans. 2020. PMID: 33170924 Review.
-
Clinical applications of detecting IgG, IgM or IgA antibody for the diagnosis of COVID-19: A meta-analysis and systematic review.Int J Infect Dis. 2021 Mar;104:415-422. doi: 10.1016/j.ijid.2021.01.016. Epub 2021 Jan 12. Int J Infect Dis. 2021. PMID: 33450372 Free PMC article.
Cited by
-
Assessing SARS-CoV-2-specific T-cell reactivity in late convalescents and vaccinees: Comparison and combination of QuantiFERON and activation-induced marker assays, and relation with antibody status.PLoS One. 2023 May 23;18(5):e0285728. doi: 10.1371/journal.pone.0285728. eCollection 2023. PLoS One. 2023. PMID: 37220145 Free PMC article.
-
Antibody Responses to mRNA COVID-19 Vaccine Among Healthcare Workers in Outpatient Clinics in Japan.Vaccines (Basel). 2025 Jan 18;13(1):90. doi: 10.3390/vaccines13010090. Vaccines (Basel). 2025. PMID: 39852868 Free PMC article.
-
Clinical usefulness of testing for severe acute respiratory syndrome coronavirus 2 antibodies.Eur J Intern Med. 2023 Jan;107:7-16. doi: 10.1016/j.ejim.2022.11.009. Epub 2022 Nov 10. Eur J Intern Med. 2023. PMID: 36379820 Free PMC article. Review.
-
Effectiveness and duration of additional immune defense provided by SARS-CoV-2 infection before and after receiving the mRNA COVID-19 vaccine BNT162b2.Vaccine X. 2024 Jun 26;19:100518. doi: 10.1016/j.jvacx.2024.100518. eCollection 2024 Aug. Vaccine X. 2024. PMID: 39040888 Free PMC article.
References
-
- WHO Coronavirus (COVID-19) Dashboard. World Health Organization. https://covid19.who.int/table.
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

