Design, immunogenicity, and efficacy of a pan-sarbecovirus dendritic-cell targeting vaccine
- PMID: 35594660
- PMCID: PMC9113741
- DOI: 10.1016/j.ebiom.2022.104062
Design, immunogenicity, and efficacy of a pan-sarbecovirus dendritic-cell targeting vaccine
Abstract
Background: There is an urgent need of a new generation of vaccine that are able to enhance protection against SARS-CoV-2 and related variants of concern (VOC) and emerging coronaviruses.
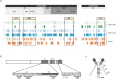
Methods: We identified conserved T- and B-cell epitopes from Spike (S) and Nucleocapsid (N) highly homologous to 38 sarbecoviruses, including SARS-CoV-2 VOCs, to design a protein subunit vaccine targeting antigens to Dendritic Cells (DC) via CD40 surface receptor (CD40.CoV2).
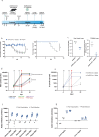
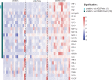
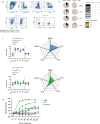
Findings: CD40.CoV2 immunization elicited high levels of cross-neutralizing antibodies against SARS-CoV-2, VOCs, and SARS-CoV-1 in K18-hACE2 transgenic mice, associated with viral control and survival after SARS-CoV-2 challenge. A direct comparison of CD40.CoV2 with the mRNA BNT162b2 vaccine showed that the two vaccines were equally immunogenic in mice. We demonstrated the potency of CD40.CoV2 to recall in vitro human multi-epitope, functional, and cytotoxic SARS-CoV-2 S- and N-specific T-cell responses that are unaffected by VOC mutations and cross-reactive with SARS-CoV-1 and, to a lesser extent, MERS epitopes.
Interpretation: We report the immunogenicity and antiviral efficacy of the CD40.CoV2 vaccine in a preclinical model providing a framework for a pan-sarbecovirus vaccine.
Fundings: This work was supported by INSERM and the Investissements d'Avenir program, Vaccine Research Institute (VRI), managed by the ANR and the CARE project funded from the Innovative Medicines Initiative 2 Joint Undertaking (JU).
Keywords: COVID-19; Pre-clinical model; SARS-CoV-2; Sarbecoviruses; Vaccine.
Copyright © 2022 The Author(s). Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
The authors S.Z., G.Z., V.G., M.C., S.C., C.L., M.S., and Y.L., are named inventors on patent applications based on this work held by Inserm Transfert. The remaining authors declare no competing interests. Inserm Transfert provided a license of CD40 targeting vaccines to biotech company LinkinVax.
Figures



References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

