Therapeutic Targeting the Allosteric Cysteinome of RAS and Kinase Families
- PMID: 35595166
- PMCID: PMC9590303
- DOI: 10.1016/j.jmb.2022.167626
Therapeutic Targeting the Allosteric Cysteinome of RAS and Kinase Families
Abstract
Allosteric mechanisms are pervasive in nature, but human-designed allosteric perturbagens are rare. The history of KRASG12C inhibitor development suggests that covalent chemistry may be a key to expanding the armamentarium of allosteric inhibitors. In that effort, irreversible targeting of a cysteine converted a non-deal allosteric binding pocket and low affinity ligands into a tractable drugging strategy. Here we examine the feasibility of expanding this approach to other allosteric pockets of RAS and kinase family members, given that both protein families are regulators of vital cellular processes that are often dysregulated in cancer and other human diseases. Moreover, these heavily studied families are the subject of numerous drug development campaigns that have resulted, sometimes serendipitously, in the discovery of allosteric inhibitors. We consequently conducted a comprehensive search for cysteines, a commonly targeted amino acid for covalent drugs, using AlphaFold-generated structures of those families. This new analysis presents potential opportunities for allosteric targeting of validated and understudied drug targets, with an emphasis on cancer therapy.
Keywords: KRAS inhibitor; allosteric inhibitor; covalent inhibitor; cysteinome; kinase inhibitor.
Copyright © 2022 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Competing Interests Statement Kenneth Westover is a member of the Scientific Advisory Board for Vibliome Therapeutics and on the Advosory Panel for Reactive Biosciences. The Westover lab receives or has received research funding from Astellas and Revolution Medicines. None of these relationships are in conflict with the content of this manuscript.
Figures
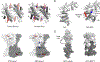

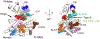
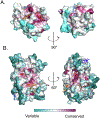
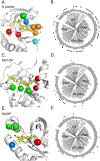

References
-
- Lu S, Shen Q, Zhang J, (2019). Allosteric Methods and Their Applications: Facilitating the Discovery of Allosteric Drugs and the Investigation of Allosteric Mechanisms. Acc. Chem. Res 52, 492–500. - PubMed
-
- Attwood MM, Fabbro D, Sokolov AV, Knapp S, Schioth HB, (2021). Trends in kinase drug discovery: targets, indications and inhibitor design. Nat. Rev. Drug Discov 20, 839–861. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

