IgA+ memory B-cells are significantly increased in patients with asthma and small airway dysfunction
- PMID: 35595320
- PMCID: PMC9630610
- DOI: 10.1183/13993003.02130-2021
IgA+ memory B-cells are significantly increased in patients with asthma and small airway dysfunction
Abstract
Background: Comprehensive studies investigated the role of T-cells in asthma which led to personalised treatment options targeting severe eosinophilic asthma. However, little is known about the contribution of B-cells to this chronic inflammatory disease. In this study we investigated the contribution of various B-cell populations to specific clinical features in asthma.
Methods: In the All Age Asthma Cohort (ALLIANCE), a subgroup of 154 adult asthma patients and 28 healthy controls were included for B-cell characterisation by flow cytometry. Questionnaires, lung function measurements, blood differential counts and allergy testing of participants were analysed together with comprehensive data on B-cells using association studies and multivariate linear models.
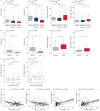
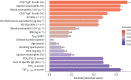
Results: Patients with severe asthma showed decreased immature B-cell populations while memory B-cells were significantly increased compared with both mild-moderate asthma patients and healthy controls. Furthermore, increased frequencies of IgA+ memory B-cells were associated with impaired lung function and specifically with parameters indicative for augmented resistance in the peripheral airways. Accordingly, asthma patients with small airway dysfunction (SAD) defined by impulse oscillometry showed increased frequencies of IgA+ memory B-cells, particularly in patients with mild-moderate asthma. Additionally, IgA+ memory B-cells significantly correlated with clinical features of SAD such as exacerbations.
Conclusions: With this study we demonstrate for the first time a significant association of increased IgA+ memory B-cells with asthma and SAD, pointing towards future options for B-cell-directed strategies in preventing and treating asthma.
Trial registration: ClinicalTrials.gov NCT02419274.
Copyright ©The authors 2022.
Conflict of interest statement
Conflict of interest: C. Happle reports grants from Novartis and Pari, outside the submitted work. M.V. Kopp reports grants from Allergopharma GmbH and Vertex GmbH; honoraria for lectures from Allergopharma GmbH, Sanofi GmbH, Infectopharm GmbH, Vertex GmbH and Leti GmbH; advisory board membership at Allergopharma GmbH and Sanofi GmbH; outside the submitted work. E. von Mutius reports royalties from Elsevier GmbH, Georg Thieme Verlag, Springer-Verlag GmbH and Elsevier Ltd; consulting fees from the Chinese University of Hong Kong, European Commission, HiPP GmbH & Co KG and AstraZeneca; lecture honoraria from Massachusetts Medical Society, Springer-Verlag GmbH, Elsevier Ltd, Boehringer Ingelheim International GmbH, European Respiratory Society, Universiteit Utrecht – Faculteit Diergeneeskunde, Universität Salzburg, Springer Medizin Verlag GmbH, Japanese Society of Pediatric Allergy and Clinical Immunology (JSPACI), Klinikum Rechts der Isar, University of Colorado, Paul-Martini-Stiftung and Imperial College London; travel support from Verein zur Förderung der Pneumologie am Krankenhaus Großhansdorf eV, Pneumologie Développement, Mondial Congress & Events GmbH & Co. KG, American Academy of Allergy, Asthma & Immunology, Imperial College London, Margaux Orange, Volkswagen Stiftung, Boehringer Ingelheim International GmbH, European Respiratory Society, Universiteit Utrecht – Faculteit Diergeneeskunde, Österreichische Gesellschaft für Allergologie und Immunologie, Massachusetts Medical Society, OM Pharma SA, Hanson Wade Ltd, iKOMM GmbH, DSI Dansk Borneastma Center, American Thoracic Society, HiPP GmbH & Co. KG and Universiteit Utrecht – Faculteit Bètawetenschappen; outside the submitted work. In addition, E. von Mutius has patent LU101064 (Barn dust extract for the prevention and treatment of diseases) pending, royalties paid to ProtectImmun for patent EP2361632 (Specific environmental bacteria for the protection from and/or the treatment of allergic, chronic inflammatory and/or autoimmune disorders, granted on 19 March 2014), and patents EP1411977 (Composition containing bacterial antigens used for the prophylaxis and the treatment of allergic diseases, granted on 18 April 2007), EP1637147 (Stable dust extract for allergy protection, granted on 10 December 2008) and EP1964570 (Pharmaceutical compound to protect against allergies and inflammatory diseases, granted on 21 November 2012) licensed to ProtectImmun. In addition, E. von Mutius is a member of the EXPANSE (funded by European Commission) Scientific Advisory Board, member of the BEAMS External Scientific Advisory Board (ESAB), member of the Editorial Board of the Journal of Allergy and Clinical Immunology: In Practice, member of the Scientific Advisory Board of the Children's Respiratory and Environmental Workgroup (CREW), member of the International Scientific and Societal Advisory Board (ISSAB) of Utrecht Life Sciences (ULS), University of Utrecht, member of the External Review Panel of the Faculty of Veterinary Science, University of Utrecht, member of the Selection Committee for the Gottfried Wilhelm Leibniz Programme (DFG), member of the International Advisory Board of the Asthma UK Centre for Applied Research (AUKCAR), member of the International Advisory Board of The Lancet Respiratory Medicine, and member of the Scientific Advisory Board of the CHILD (Canadian Healthy Infant Longitudinal Development) study, McMaster University, Hamilton, Canada. T. Bahmer reports grants from Network University Medicine (NUM): National Pandemic Cohort Network (NAPKON); consulting fees and lecture honoraria from AstraZeneca, Novartis, GlaxoSmithKline, Roche and Chiesi; travel support from Chiesi and AstraZeneca; outside the submitted work. K.F. Rabe reports lecture honoraria from AstraZeneca, Boehringer Ingelheim, Chiesi Pharmaceuticals, Novartis, Sanofi Regeneron, GlaxoSmithKline, Berlin-Chemie and Roche; advisory board membership at AstraZeneca and Sanofi Regeneron; leadership roles with the German Center for Lung Research (DZL), German Chest Society (DGP) and American Thoracic Society; outside the submitted work. A. Meyer-Bahlburg reports lecture honoraria from Pfizer; travel support from CSL Behring; advisory board membership with Pfizer; outside the submitted work. G. Hansen reports consulting fees from Sanofi GmbH; lecture honoraria from MedUpdate and AbbVie; outside the submitted work. All other authors have nothing to disclose.
Figures