Leukotriene receptor antagonists and risk of neuropsychiatric events in children, adolescents and young adults: a self-controlled case series
- PMID: 35595323
- PMCID: PMC9630607
- DOI: 10.1183/13993003.02467-2021
Leukotriene receptor antagonists and risk of neuropsychiatric events in children, adolescents and young adults: a self-controlled case series
Abstract
Background: Leukotriene receptor antagonists (LTRAs) are widely used for asthma and allergic rhinitis (AR), but concerns about the risk of neuropsychiatric events (NPEs) have been raised since the first Drug Safety Communication by the US Food and Drug Administration in 2008. This study evaluates the association between LTRA use and NPEs in children, adolescents and young adults with asthma or AR.
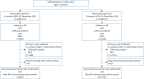
Methods: A self-controlled case series study was conducted using the Korean National Health Insurance Service claims database from two 3-year observation periods (observation period 1 (Obs1): 2005-2007; observation period 2 (Obs2): 2016-2018). Asthma or AR patients aged 3-30 years who were prescribed LTRAs and diagnosed with NPEs were included. The incidence rate ratios (IRRs) for the exposed period and risk periods (1-3, 4-7, 8-14, 15-30, 31-90 and >90 days from initiation of LTRA) compared with unexposed periods were calculated using conditional Poisson regression. Subgroup analysis according to age group, type of NPEs and indication of LTRA was performed.
Results: Among 17 001 included patients, the risk of NPEs increased in Obs2 (IRR 1.11, 95% CI 1.00-1.22), but did not increase in Obs1. Risk was increased during risk periods 4-7 days (IRR 2.36, 95% CI 1.99-2.76) and 8-14 days (IRR 1.78, 95% CI 1.46-2.15) after initiation of LTRA, particularly in adolescents (IRR 1.28, 95% CI 1.05-1.55) and young adults (IRR 1.14, 95% CI 1.02-1.28), while risk was decreased in children (3-11 years). Risk was not increased for any single type of NPE. AR patients were at increased risk (IRR 1.19, 95% CI 1.01-1.39), but not those with asthma.
Conclusions: Overall, risk of NPEs with LTRA use differed between risk periods and subgroups. Physicians should prescribe LTRAs according to indications and inform patients about possible NPEs.
Copyright ©The authors 2022.
Conflict of interest statement
Conflict of interest: J.S. Park has nothing to disclose. Y.J. Cho has nothing to disclose. J-Y. Yun reports lecture fees from the Korean Society for Human Brain Mapping, outside the submitted work. H.J. Lee has nothing to disclose. J. Yu has nothing to disclose. H-J. Yang reports lecture fees from the Korean Academy of Pediatric Allergy and Respiratory Disease (KAPARD), Korea Disease Control and Prevention Agency, and Korea National Enterprise for Clinical Trials, outside the submitted work. D.I. Suh reports research grants from KAPARD for the submitted work and lecture fees from KAPARD, Organon Korea and Sama Pharm, outside the submitted work.
Figures

References
-
- Global Initiative for Asthma (GINA) . Global Strategy for Asthma Management and Prevention. 2021. Available from: http://ginasthma.org/
-
- US Food and Drug Administration . Updated information on leukotriene inhibitors: montelukast (marketed as Singulair), zafirlukast (marketed as Accolate), and zileuton (marketed as Zyflo and Zyflo CR). 2009. https://wayback.archive-it.org/7993/*/https://www.fda.gov/Drugs/DrugSafe... Date last accessed: 29 April 2022.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
