The β-Hydroxybutyrate-GPR109A Receptor Regulates Fasting-induced Plasticity in the Mouse Adrenal Medulla
- PMID: 35595517
- PMCID: PMC9188660
- DOI: 10.1210/endocr/bqac077
The β-Hydroxybutyrate-GPR109A Receptor Regulates Fasting-induced Plasticity in the Mouse Adrenal Medulla
Abstract
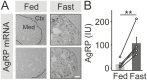
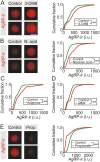
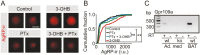
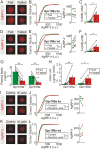
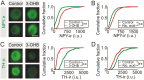
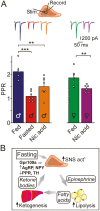
During fasting, increased sympathoadrenal activity leads to epinephrine release and multiple forms of plasticity within the adrenal medulla including an increase in the strength of the preganglionic → chromaffin cell synapse and elevated levels of agouti-related peptide (AgRP), a peptidergic cotransmitter in chromaffin cells. Although these changes contribute to the sympathetic response, how fasting evokes this plasticity is not known. Here we report these effects involve activation of GPR109A (HCAR2). The endogenous agonist of this G protein-coupled receptor is β-hydroxybutyrate, a ketone body whose levels rise during fasting. In wild-type animals, 24-hour fasting increased AgRP-ir in adrenal chromaffin cells but this effect was absent in GPR109A knockout mice. GPR109A agonists increased AgRP-ir in isolated chromaffin cells through a GPR109A- and pertussis toxin-sensitive pathway. Incubation of adrenal slices in nicotinic acid, a GPR109A agonist, mimicked the fasting-induced increase in the strength of the preganglionic → chromaffin cell synapse. Finally, reverse transcription polymerase chain reaction experiments confirmed the mouse adrenal medulla contains GPR109A messenger RNA. These results are consistent with the activation of a GPR109A signaling pathway located within the adrenal gland. Because fasting evokes epinephrine release, which stimulates lipolysis and the production of β-hydroxybutyrate, our results indicate that chromaffin cells are components of an autonomic-adipose-hepatic feedback circuit. Coupling a change in adrenal physiology to a metabolite whose levels rise during fasting is presumably an efficient way to coordinate the homeostatic response to food deprivation.
Keywords: AgRP; GPR109A; adrenal; autonomic nervous system; fasting; ketone bodies.
© The Author(s) 2022. Published by Oxford University Press on behalf of the Endocrine Society. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Figures
Similar articles
-
AgRP-Expressing Adrenal Chromaffin Cells Are Involved in the Sympathetic Response to Fasting.Endocrinology. 2017 Aug 1;158(8):2572-2584. doi: 10.1210/en.2016-1268. Endocrinology. 2017. PMID: 28531318 Free PMC article.
-
Fasting induces a form of autonomic synaptic plasticity that prevents hypoglycemia.Proc Natl Acad Sci U S A. 2016 May 24;113(21):E3029-38. doi: 10.1073/pnas.1517275113. Epub 2016 Apr 18. Proc Natl Acad Sci U S A. 2016. PMID: 27092009 Free PMC article.
-
Differential Modulation of Catecholamine and Adipokine Secretion by the Short Chain Fatty Acid Receptor FFAR3 and α2-Adrenergic Receptors in PC12 Cells.Int J Mol Sci. 2024 May 11;25(10):5227. doi: 10.3390/ijms25105227. Int J Mol Sci. 2024. PMID: 38791266 Free PMC article.
-
Selective neural regulation of epinephrine and norepinephrine cells in the adrenal medulla -- cardiovascular implications.Clin Exp Hypertens. 1996 Aug;18(6):731-51. doi: 10.3109/10641969609081778. Clin Exp Hypertens. 1996. PMID: 8842561 Review.
-
Muscarinic receptors in adrenal chromaffin cells: physiological role and regulation of ion channels.Pflugers Arch. 2018 Jan;470(1):29-38. doi: 10.1007/s00424-017-2047-2. Epub 2017 Jul 31. Pflugers Arch. 2018. PMID: 28762161 Review.
Cited by
-
Unlocking the role of lactate: metabolic pathways, signaling, and gene regulation in postmitotic retinal cells.Front Ophthalmol (Lausanne). 2023 Nov 8;3:1296624. doi: 10.3389/fopht.2023.1296624. eCollection 2023. Front Ophthalmol (Lausanne). 2023. PMID: 38983010 Free PMC article. Review.
-
Ketone Body Induction: Insights into Metabolic Disease Management.Biomedicines. 2025 Jun 16;13(6):1484. doi: 10.3390/biomedicines13061484. Biomedicines. 2025. PMID: 40564203 Free PMC article. Review.
References
-
- Cahill GF Jr. Fuel metabolism in starvation. Annu Rev Nutr. 2006;26:1-22. - PubMed

