Insights into the developing fovea revealed by imaging
- PMID: 35595637
- PMCID: PMC12183701
- DOI: 10.1016/j.preteyeres.2022.101067
Insights into the developing fovea revealed by imaging
Abstract
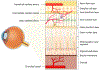
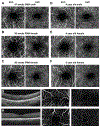
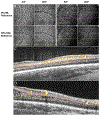
Early development of the fovea has been documented by histological studies over the past few decades. However, structural distortion due to sample processing and the paucity of high-quality post-mortem tissue has limited the effectiveness of this approach. With the continuous progress in high-resolution non-invasive imaging technology, most notably optical coherence tomography (OCT) and OCT angiography (OCT-A), in vivo visualization of the developing retina has become possible. Combining the information from histologic studies with this novel imaging information has provided a more complete and accurate picture of retinal development, and in particular the developing fovea. Advances in neonatal care have increased the survival rate of extremely premature infants. However, with enhanced survival there has been an attendant increase in retinal developmental complications. Several key abnormalities, including a thickening of the inner retina at the foveal center, a shallower foveal pit, a smaller foveal avascular zone, and delayed development of the photoreceptors have been described in preterm infants when compared to full-term infants. Notably these abnormalities, which are consistent with a partial arrest of foveal development, appear to persist into later childhood and adulthood in these eyes of individuals born prematurely. Understanding normal foveal development is vital to interpreting these pathologic findings associated with prematurity. In this review, we first discuss the various advanced imaging technologies that have been adapted for imaging the infant eye. We then review the key events and steps in the development of the normal structure of the fovea and contrast structural features in normal and preterm retina from infancy to childhood. Finally, we discuss the development of the perifoveal retinal microvasculature and highlight future opportunities to expand our understanding of the developing fovea.
Keywords: Developing fovea; Infant imaging; Optical coherence tomography; Optical coherence tomography angiography; Prematurity.
Copyright © 2022. Published by Elsevier Ltd.
Conflict of interest statement
Declaration of competing interest None.
Figures





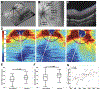
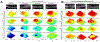

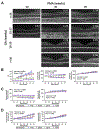
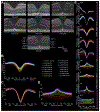





Similar articles
-
Delayed macular development in preterm infants with spontaneously regressed retinopathy of prematurity.BMC Ophthalmol. 2025 Jan 17;25(1):27. doi: 10.1186/s12886-025-03867-6. BMC Ophthalmol. 2025. PMID: 39833754 Free PMC article.
-
Different corticosteroids and regimens for accelerating fetal lung maturation for babies at risk of preterm birth.Cochrane Database Syst Rev. 2022 Aug 9;8(8):CD006764. doi: 10.1002/14651858.CD006764.pub4. Cochrane Database Syst Rev. 2022. PMID: 35943347 Free PMC article.
-
Maternal and neonatal outcomes of elective induction of labor.Evid Rep Technol Assess (Full Rep). 2009 Mar;(176):1-257. Evid Rep Technol Assess (Full Rep). 2009. PMID: 19408970 Free PMC article.
-
Early (< 8 days) systemic postnatal corticosteroids for prevention of bronchopulmonary dysplasia in preterm infants.Cochrane Database Syst Rev. 2017 Oct 24;10(10):CD001146. doi: 10.1002/14651858.CD001146.pub5. Cochrane Database Syst Rev. 2017. Update in: Cochrane Database Syst Rev. 2021 Oct 21;10:CD001146. doi: 10.1002/14651858.CD001146.pub6. PMID: 29063585 Free PMC article. Updated.
-
Effects of targeting lower versus higher arterial oxygen saturations on death or disability in preterm infants.Cochrane Database Syst Rev. 2017 Apr 11;4(4):CD011190. doi: 10.1002/14651858.CD011190.pub2. Cochrane Database Syst Rev. 2017. PMID: 28398697 Free PMC article.
Cited by
-
Congenital aniridia beyond black eyes: From phenotype and novel genetic mechanisms to innovative therapeutic approaches.Prog Retin Eye Res. 2023 Jul;95:101133. doi: 10.1016/j.preteyeres.2022.101133. Epub 2022 Oct 22. Prog Retin Eye Res. 2023. PMID: 36280537 Free PMC article. Review.
-
Subclinical structural atypicality of retinal thickness and its association with gray matter volume in the visual cortex of maltreated children.Sci Rep. 2024 May 20;14(1):11465. doi: 10.1038/s41598-024-62392-6. Sci Rep. 2024. PMID: 38769421 Free PMC article.
-
Longitudinal Assessment of OCT-Based Measures of Foveal Cone Structure in Achromatopsia.Invest Ophthalmol Vis Sci. 2024 Apr 1;65(4):16. doi: 10.1167/iovs.65.4.16. Invest Ophthalmol Vis Sci. 2024. PMID: 38587442 Free PMC article.
-
Clinical Utilisation of Wide-Field Optical Coherence Tomography and Angiography: A Narrative Review.Ophthalmol Ther. 2024 Apr;13(4):903-915. doi: 10.1007/s40123-024-00905-2. Epub 2024 Feb 19. Ophthalmol Ther. 2024. PMID: 38372953 Free PMC article. Review.
-
Optical Coherence Tomography and Optical Coherence Tomography Angiography in Pediatric Retinal Diseases.Diagnostics (Basel). 2023 Apr 18;13(8):1461. doi: 10.3390/diagnostics13081461. Diagnostics (Basel). 2023. PMID: 37189561 Free PMC article. Review.
References
-
- Akerblom H, Larsson E, Eriksson U, Holmstrom G, 2011. Central macular thickness is correlated with gestational age at birth in prematurely born children. Br. J. Ophthalmol 95, 799–803. - PubMed
-
- Akula JD, Hansen RM, Martinez-Perez ME, Fulton AB, 2007. Rod photoreceptor function predicts blood vessel abnormality in retinopathy of prematurity. Invest. Ophthalmol. Vis. Sci 48, 4351–4359. - PubMed
-
- Alabduljalil T, Westall CA, Reginald A, Farsiu S, Chiu SJ, Arshavsky A, Toth CA, Lam WC, 2019. Demonstration of anatomical development of the human macula within the first 5 years of life using handheld OCT. Int. Ophthalmol 39, 1533–1542. - PubMed
-
- Allingham MJ, Cabrera MT, O’Connell RV, Maldonado RS, Tran-Viet D, Toth CA, Freedman SF, El-Dairi MA, 2013. Racial variation in optic nerve head parameters quantified in healthy newborns by handheld spectral domain optical coherence tomography. J. Am. Assoc. Pediatr. Ophthalmol. Strabismus 17, 501–506. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

