First purified recombinant CYP75B including transmembrane helix with unexpected high substrate specificity to (2R)-naringenin
- PMID: 35595763
- PMCID: PMC9122903
- DOI: 10.1038/s41598-022-11556-3
First purified recombinant CYP75B including transmembrane helix with unexpected high substrate specificity to (2R)-naringenin
Abstract
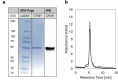
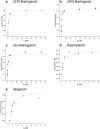
Anthochlor pigments (chalcones and aurones) play an important role in yellow flower colourization, the formation of UV-honey guides and show numerous health benefits. The B-ring hydroxylation of chalcones is performed by membrane bound cytochrome P450 enzymes. It was assumed that usual flavonoid 3'-hydroxlases (F3'Hs) are responsible for the 3,4- dihydroxy pattern of chalcones, however, we previously showed that a specialized F3'H, namely chalcone 3-hydroxylase (CH3H), is necessary for the hydroxylation of chalcones. In this study, a sequence encoding membrane bound CH3H from Dahlia variabilis was recombinantly expressed in yeast and a purification procedure was developed. The optimized purification procedure led to an overall recovery of 30% recombinant DvCH3H with a purity of more than 84%. The enzyme was biochemically characterized with regard to its kinetic parameters on various substrates, including racemic naringenin, as well as its enantiomers (2S)-, and (2R)-naringenin, apigenin and kaempferol. We report for the first time the characterization of a purified Cytochrome P450 enzyme from the flavonoid biosynthesis pathway, including the transmembrane helix. Further, we show for the first time that recombinant DvCH3H displays a higher affinity for (2R)-naringenin than for (2S)-naringenin, although (2R)-flavanones are not naturally formed by chalcone isomerase.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



Similar articles
-
Allelic variants from Dahlia variabilis encode flavonoid 3'-hydroxylases with functional differences in chalcone 3-hydroxylase activity.Arch Biochem Biophys. 2010 Feb 1;494(1):40-5. doi: 10.1016/j.abb.2009.11.015. Epub 2009 Nov 18. Arch Biochem Biophys. 2010. PMID: 19931222
-
Cloning, functional expression, and characterization of a chalcone 3-hydroxylase from Cosmos sulphureus.J Exp Bot. 2010 Jul;61(12):3451-9. doi: 10.1093/jxb/erq169. Epub 2010 Jun 21. J Exp Bot. 2010. PMID: 20566567
-
Structural and Interactional Analysis of the Flavonoid Pathway Proteins: Chalcone Synthase, Chalcone Isomerase and Chalcone Isomerase-like Protein.Int J Mol Sci. 2024 May 22;25(11):5651. doi: 10.3390/ijms25115651. Int J Mol Sci. 2024. PMID: 38891840 Free PMC article.
-
Flower colour and cytochromes P450.Philos Trans R Soc Lond B Biol Sci. 2013 Jan 6;368(1612):20120432. doi: 10.1098/rstb.2012.0432. Print 2013 Feb 19. Philos Trans R Soc Lond B Biol Sci. 2013. PMID: 23297355 Free PMC article. Review.
-
Comparative Molecular Mechanisms of Biosynthesis of Naringenin and Related Chalcones in Actinobacteria and Plants: Relevance for the Obtention of Potent Bioactive Metabolites.Antibiotics (Basel). 2022 Jan 10;11(1):82. doi: 10.3390/antibiotics11010082. Antibiotics (Basel). 2022. PMID: 35052959 Free PMC article. Review.
References
-
- Halbwirth H, Muster G, Stich K. Unraveling the biochemical base of dahlia flower coloration. Nat. Prod. Commun. 2008;3:1934578X0800300807. doi: 10.1177/1934578x0800300807. - DOI
-
- McClaren B. Encyclopedia of Dahlias. Timber Press; 2009.
-
- Giannasi DE. The Flavonoid Systematics of the Genus Dahlia (Compositae) New York Botanical Garden; 1975.
-
- Broertjes C, Ballego JM. Mutation breeding of Dahlia variabilis. Euphytica. 1967;16:171–176. doi: 10.1007/bf00043451. - DOI
-
- Giannasi DE. Flavonoid chemistry and evolution in dahlia (compositae) Bull. Torrey Bot. Club. 1975;102:404–412. doi: 10.2307/2484767. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

