Platelet-Mediated Transfer of Cardioprotection by Remote Ischemic Conditioning and Its Abrogation by Aspirin But Not by Ticagrelor
- PMID: 35595877
- PMCID: PMC10517043
- DOI: 10.1007/s10557-022-07345-9
Platelet-Mediated Transfer of Cardioprotection by Remote Ischemic Conditioning and Its Abrogation by Aspirin But Not by Ticagrelor
Abstract
Purpose: The role of platelets during myocardial ischemia/reperfusion (I/R) is ambivalent. They contribute to injury but also to cardioprotection. Repeated blood flow restriction and reperfusion in a tissue/organ remote from the heart (remote ischemic conditioning, RIC) reduce myocardial I/R injury and attenuate platelet activation. Whether or not platelets mediate RIC's cardioprotective signal is currently unclear.
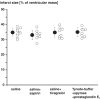
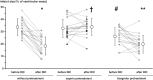
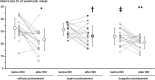
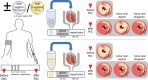
Methods and results: Venous blood from healthy volunteers (without or with pretreatment of 500/1000 mg aspirin or 180 mg ticagrelor orally, 2-3 h before the study, n = 18 each) was collected before and after RIC (3 × 5 min blood pressure cuff inflation at 200 mmHg on the left upper arm/5 min deflation). Washed platelets were isolated. Platelet-poor plasma was used to prepare plasma-dialysates. Platelets (25 × 103/µL) or plasma-dialysates (1:10) prepared before and after RIC from untreated versus aspirin- or ticagrelor-pretreated volunteers, respectively, were infused into isolated buffer-perfused rat hearts. Hearts were subjected to global 30 min/120 min I/R. Infarct size was stained. Infarct size was less with infusion of platelets/plasma-dialysate after RIC (18 ± 7%/23 ± 9% of ventricular mass) than with platelets/plasma-dialysate before RIC (34 ± 7%/33 ± 8%). Aspirin pretreatment abrogated the transfer of RIC's cardioprotection by platelets (after/before RIC, 34 ± 7%/33 ± 7%) but only attenuated that by plasma-dialysate (after/before RIC, 26 ± 8%/32 ± 5%). Ticagrelor pretreatment induced an in vivo formation of cardioprotective factor(s) per se (platelets/plasma-dialysate before RIC, 26 ± 7%/26 ± 7%) but did not impact on RIC's cardioprotection by platelets/plasma-dialysate (20 ± 7%/21 ± 5%).
Conclusion: Platelets serve as carriers for RIC's cardioprotective signal through an aspirin-sensitive and thus cyclooxygenase-dependent mechanism. The P2Y12 inhibitor ticagrelor per se induces a humoral cardioprotective signal.
Keywords: Aspirin; Cardioprotection; Ischemia/reperfusion; Remote ischemic conditioning; Ticagrelor.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
Bioassays of Humoral Cardioprotective Factors Released by Remote Ischemic Conditioning in Patients Undergoing Coronary Artery Bypass Surgery.J Cardiovasc Pharmacol Ther. 2022 Jan-Dec;27:10742484221097273. doi: 10.1177/10742484221097273. J Cardiovasc Pharmacol Ther. 2022. PMID: 35510644
-
Plasma from remotely conditioned pigs reduces infarct size when given before or after ischemia to isolated perfused rat hearts.Pflugers Arch. 2019 Dec;471(11-12):1371-1379. doi: 10.1007/s00424-019-02314-y. Epub 2019 Oct 21. Pflugers Arch. 2019. PMID: 31631252
-
Remote ischemic conditioning in Ossabaw minipigs induces the release of humoral cardioprotective triggers, but the myocardium does not respond with reduced infarct size.Am J Physiol Heart Circ Physiol. 2022 Dec 1;323(6):H1365-H1375. doi: 10.1152/ajpheart.00580.2022. Epub 2022 Nov 11. Am J Physiol Heart Circ Physiol. 2022. PMID: 36367697 Free PMC article.
-
Remote ischemic conditioning: the cardiologist's perspective.J Cardiovasc Med (Hagerstown). 2012 Nov;13(11):667-74. doi: 10.2459/JCM.0b013e328357bff2. J Cardiovasc Med (Hagerstown). 2012. PMID: 23114270 Review.
-
Remote ischemic conditioning.J Am Coll Cardiol. 2015 Jan 20;65(2):177-95. doi: 10.1016/j.jacc.2014.10.031. J Am Coll Cardiol. 2015. PMID: 25593060 Free PMC article. Review.
Cited by
-
The spleen in ischaemic heart disease.Nat Rev Cardiol. 2025 Jul;22(7):497-509. doi: 10.1038/s41569-024-01114-x. Epub 2025 Jan 2. Nat Rev Cardiol. 2025. PMID: 39743566 Review.
-
Neuroprotective effect of remote ischemic preconditioning in patients undergoing cardiac surgery: A randomized controlled trial.Front Cardiovasc Med. 2022 Sep 6;9:952033. doi: 10.3389/fcvm.2022.952033. eCollection 2022. Front Cardiovasc Med. 2022. PMID: 36148077 Free PMC article.
-
Preclinical multi-target strategies for myocardial ischemia-reperfusion injury.Front Cardiovasc Med. 2022 Aug 22;9:967115. doi: 10.3389/fcvm.2022.967115. eCollection 2022. Front Cardiovasc Med. 2022. PMID: 36072870 Free PMC article. Review.
-
Low-dose and standard-dose ticagrelor compared with clopidogrel in patients with acute coronary syndromes: A cohort study from china.Front Cardiovasc Med. 2022 Jul 26;9:937261. doi: 10.3389/fcvm.2022.937261. eCollection 2022. Front Cardiovasc Med. 2022. PMID: 35958420 Free PMC article.
-
Imatinib attenuates reperfusion injury in a rat model of acute myocardial infarction.Basic Res Cardiol. 2023 Jan 13;118(1):2. doi: 10.1007/s00395-022-00974-z. Basic Res Cardiol. 2023. PMID: 36639597 Free PMC article.
References
-
- Szummer K, Wallentin L, Lindhagen L, et al. Improved outcomes in patients with ST-elevation myocardial infarction during the last 20 years are related to implementation of evidence-based treatments: experiences from the SWEDEHEART registry 1995–2014. Eur Heart J. 2017;38:3056–3065. doi: 10.1093/eurheartj/ehx515. - DOI - PMC - PubMed
-
- Heusch G, Gersh BJ. The pathophysiology of acute myocardial infarction and strategies of protection beyond reperfusion: a continual challenge. Eur Heart J. 2017;38:774–784. - PubMed
-
- Valgimigli M, Bueno H, Byrne RA, et al. 2017 ESC focused update on dual antiplatelet therapy in coronary artery disease developed in collaboration with EACTS: the task force for dual antiplatelet therapy in coronary artery disease of the European Society of Cardiology (ESC) and of the European Association for Cardio-Thoracic Surgery (EACTS) Eur Heart J. 2018;39:213–260. doi: 10.1093/eurheartj/ehx419. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

