Cost Effectiveness of Inclisiran in Atherosclerotic Cardiovascular Patients with Elevated Low-Density Lipoprotein Cholesterol Despite Statin Use: A Threshold Analysis
- PMID: 35595929
- PMCID: PMC9468070
- DOI: 10.1007/s40256-022-00534-9
Cost Effectiveness of Inclisiran in Atherosclerotic Cardiovascular Patients with Elevated Low-Density Lipoprotein Cholesterol Despite Statin Use: A Threshold Analysis
Abstract
Background: Inclisiran is a novel, cholesterol-lowering therapy, with a long duration of effect, administered every 6 months (subcutaneously by a healthcare professional). In the ORION-10 trial in US patients with atherosclerotic cardiovascular disease (ASCVD) in addition to maximum tolerated statins, with or without ezetimibe, inclisiran demonstrated statistically significant reductions in low-density lipoprotein cholesterol (LDL-C) of up to 51%. This is the first peer-reviewed publication to investigate the price at which inclisiran is cost effective in the US.
Objective: The aim of this study was to determine the maximum price at which inclisiran is cost effective in addition to standard of care, in US patients with ASCVD, versus standard of care alone, at different willingness-to-pay thresholds.
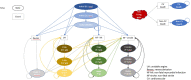
Design, setting and participants: A lifetime Markov model from the US health system perspective, including 15 health states, was used to evaluate the cost effectiveness of inclisiran. The following states were separated by time from a previous cardiovascular event (0-1 years, 1-2 years, 2+ years ['stable']): initial, unstable angina, myocardial infarction, and stroke. Additional states included revascularization and death (cardiovascular or non-cardiovascular causes). Baseline risk of cardivoascular events were from US database sources or published literature. Reductions in LDL-C from inclisiran were from the ORION-10 trial. LDL-C reduction was used to adjust baseline risk of cardiovascular events, based on established relationships between 1 mmol/L reduction in LDL-C and decreases in cardiovascular events, from the Cholesterol Treatment Trialists studies. The population included adults with a history of ASCVD, and LDL-C ≥ 70 mg/dL, despite maximum tolerated doses of statin therapy.
Interventions: Inclisiran as an adjunct to standard of care, compared with standard of care alone.
Main outcomes and measures: The threshold price of inclisiran.
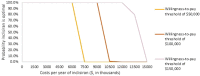
Results: Inclisiran as an adjunct to standard of care resulted in threshold annual inclisiran prices of $6383, $9973, and $13,563 at willingness-to-pay thresholds of $50,000, $100,000, and $150,000 per quality-adjusted life-year, respectively. Probabilistic sensitivity analysis showed that at a threshold of $100,000 per QALY, inclisiran had a 100% probability of being cost effective, with an annual price below $9000. At the publicly available price of $3250 per dose, inclisiran was found to have an incremental cost-effectiveness ratio just above the $50,000 per QALY threshold, of $51,686.
Conclusions and relevance: This study identified the price at which inclisiran is cost effective for the US health system, at generally accepted willingness-to-pay thresholds.
© 2022. The Author(s).
Conflict of interest statement
Caresse Campbell, Batul Electricwala, and Joaquim Cristino are employees of Novartis Pharmaceuticals Corporation, USA, and shareholders of Novartis Pharmaceuticals Corporation stock. Nihar R. Desai is an employee of Yale University School of Medicine and works under contract with the Centers for Medicare and Medicaid Services to develop and maintain performance measures used for public reporting and pay-for-performance programs. Nihar R. Desai also reports research grants and consulting for Amgen, Astra Zeneca, Boehringer Ingelheim, Cytokinetics, MyoKardia, Relypsa, Novartis, and scPharmaceuticals. Margaret Petrou, David Trueman, and Fionn Woodcock are employees of Source Health Economics, UK, the health economics and outcomes research (HEOR) company that conducted the cost-effectiveness analysis and provided writing services. Work by Source Health Economics was funded by Novartis. Inclisiran, sold under the brand name Leqvio®, has been developed by Novartis and is approved in Europe for lowering LDL-cholesterol levels in patients with hypercholesterolemia or mixed dyslipidemia. The efficacy and safety of inclisiran has been evaluated in the ORION clinical trial program; ORION clinical trials cited in this manuscript were developed and funded by Novartis. Caresse Campbell and Margaret Petrou had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Figures
References
-
- Heron MP. Deaths: leading causes for 2013. Natl Vital Stat Rep. 2016;65(2):1–95. - PubMed
-
- Kaiser Permanente. Atherosclerotic Cardiovascular Disease (ASCVD) Primary Prevention Guideline. October 2020. https://wa.kaiserpermanente.org/static/pdf/public/guidelines/ascvd-prima.... Accessed 4 Feb 2021.
-
- Ference BA, Ginsberg HN, Graham I, et al. Low-density lipoproteins cause atherosclerotic cardiovascular disease. 1. Evidence from genetic, epidemiologic, and clinical studies. A consensus statement from the European Atherosclerosis Society Consensus Panel. Eur Heart J. 2017;38(32):2459–2472. - PMC - PubMed
-
- Abdullah SM, Defina LF, Leonard D, et al. Long-term association of low-density lipoprotein cholesterol with cardiovascular mortality in individuals at low 10-year risk of atherosclerotic cardiovascular disease: results from the Cooper Center Longitudinal Study. Circulation. 2018;138(21):2315–2325. doi: 10.1161/CIRCULATIONAHA.118.034273. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

