Loss of RTN3 phenocopies chronic kidney disease and results in activation of the IGF2-JAK2 pathway in proximal tubular epithelial cells
- PMID: 35596061
- PMCID: PMC9166791
- DOI: 10.1038/s12276-022-00763-7
Loss of RTN3 phenocopies chronic kidney disease and results in activation of the IGF2-JAK2 pathway in proximal tubular epithelial cells
Abstract
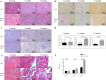
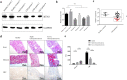
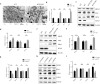
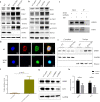
Reticulon 3 (RTN3) is an endoplasmic reticulum protein that has previously been shown to play roles in neurodegenerative diseases, but little is known about its function in the kidneys. The aim of the present study was to clarify the roles of RTN3 in chronic kidney disease (CKD) and kidney fibrosis. In this study, RTN3 levels were measured in kidney tissues from healthy controls and CKD or kidney fibrosis patients. An RTN3-null mouse model was generated to explore the pathophysiological roles of RTN3 in the kidneys. The underlying mechanisms were studied in primary proximal tubular epithelial cells and HEK293 cells in vitro. The results showed that (1) a reduction in RTN3 in mice induces CKD and kidney fibrosis; (2) decreased RTN3 expression is found in patients with CKD; (3) RTN3 plays critical roles in regulating collagen biosynthesis and mitochondrial function; and (4) mechanistically, RTN3 regulates these phenotypes by interacting with GC-Rich Promoter Binding Protein 1 (GPBP1), which activates the IGF2-JAK2-STAT3 pathway. Our study indicates that RTN3 might play crucial roles in CKD and kidney fibrosis and that a reduction in RTN3 in the kidneys might be a risk factor for CKD and kidney fibrosis.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

