Protocol of a randomized, double-blind, placebo-controlled study of the effect of probiotics on the gut microbiome of patients with gastro-oesophageal reflux disease treated with rabeprazole
- PMID: 35596146
- PMCID: PMC9123715
- DOI: 10.1186/s12876-022-02320-y
Protocol of a randomized, double-blind, placebo-controlled study of the effect of probiotics on the gut microbiome of patients with gastro-oesophageal reflux disease treated with rabeprazole
Abstract
Background: For patients with gastro-oesophageal reflux symptoms, the preferred treatment is proton pump inhibitor (PPI) administration for approximately 8 weeks. However, long-term use of PPIs can cause gut microbiome (GM) disturbances. This study is designed to evaluate the effect of probiotics combined with a PPI on the GM and gastrointestinal symptoms of patients with gastro-oesophageal reflux disease (GERD).
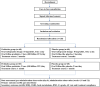
Method: This is a randomized, double-blind, placebo-controlled trial. A total of 120 eligible patients with GERD will be randomized into the experimental group or the control group. The treatment includes two phases: the initial treatment period lasts 8 weeks (weeks 1-8), and the maintenance treatment period lasts 4 weeks (weeks 9-12). During the initial treatment period, the experimental group will take rabeprazole and LiHuo probiotics, and the control group will take rabeprazole and a probiotic placebo; during the maintenance treatment period, the experimental group will take LiHuo probiotics, and the control group will take a probiotic placebo. The primary measure is the change in the GM. The secondary measures are the Reflux Disease Questionnaire (RDQ) score, Gastrointestinal Symptom Rating Scale (GSRS) score, faecal metabolome (FM), body mass index, Los Angeles grade of oesophagitis, adverse event (AE) rate and treatment compliance. Each outcome indicator will be assessed at day 0 (before administration), day 28 and/or 56 (during administration), and day 84 (end of administration) to reveal intragroup differences. AEs will be monitored to assess the safety of LiHuo probiotics.
Discussion: This will be the first trial to use the intestinal flora metagene method to analyse the effects of probiotics on patients with GERD receiving long-term PPI treatment. The goal is to provide evidence for the use of probiotics to reduce intestinal flora disorders and other symptoms of gastrointestinal discomfort in patients with GERD who have used PPIs for a long period.
Trial registration: Chinese Clinical Trial Registry (ChiCTR) (NO. ChiCTR2000038409). Registered on November 22, 2020, http://www.chictr.org.cn/showproj.aspx?proj=56358 .
Keywords: GERD; Gut microbiome; Probiotics; Proton pump inhibitors.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests. The study has not received funding/assistance from a commercial organization.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical