Mir-675-5p supports hypoxia-induced drug resistance in colorectal cancer cells
- PMID: 35596172
- PMCID: PMC9123752
- DOI: 10.1186/s12885-022-09666-2
Mir-675-5p supports hypoxia-induced drug resistance in colorectal cancer cells
Abstract
Background: The uncontrolled proliferation of cancer cells determines hypoxic conditions within the neoplastic mass with consequent activation of specific molecular pathways that allow cells to survive despite oxygen deprivation. The same molecular pathways are often the cause of chemoresistance. This study aims to investigate the role of the hypoxia-induced miR-675-5p in 5-Fluorouracil (5-FU) resistance on colorectal cancer (CRC) cells.
Methods: CRC cell lines were treated with 5-Fu and incubated in normoxic or hypoxic conditions; cell viability has been evaluated by MTT assay. MiR-675-5p levels were analysed by RT-PCR and loss and gain expression of the miRNA has been obtained by the transfection of miRNA antagomir or miRNA mimic. Total protein expression of different apoptotic markers was analysed through western blot assay. MirWalk 2.0 database search engine was used to investigate the putative targets of the miR-675-5p involved in the apoptotic process. Finally, the luciferase assay was done to confirm Caspase-3 as a direct target of the miR-675-5p.
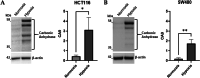
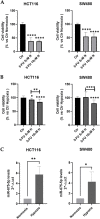
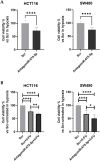
Results: Our data demonstrated that hypoxia-induced miR-675-5p counteracts the apoptotic signal induced by 5-FU, thus taking part in the drug resistance response. We showed that the apoptotic markers, cleaved PARP and cleaved caspase-3, increased combining miR-675-5p inhibition with 5-FU treatment. Moreover, we identified pro-caspase-3 among the targets of the miR-675-5p.
Conclusion: Our data demonstrate that the inhibition of hypoxia-induced miR-675-5p combined with 5-FU treatment can enhances drug efficacy in both prolonged hypoxia and normoxia, indicating a possible strategy to partially overcome chemoresistance.
Keywords: 5-fluorouracil (5-FU); Apoptosis; Colorectal cancer (CRC); Drug resistance; Hypoxia; MicroRNA.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests
Figures



References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

