Celastrol mitigates inflammation in sepsis by inhibiting the PKM2-dependent Warburg effect
- PMID: 35596191
- PMCID: PMC9121578
- DOI: 10.1186/s40779-022-00381-4
Celastrol mitigates inflammation in sepsis by inhibiting the PKM2-dependent Warburg effect
Abstract
Background: Sepsis involves life-threatening organ dysfunction and is caused by a dysregulated host response to infection. No specific therapies against sepsis have been reported. Celastrol (Cel) is a natural anti-inflammatory compound that shows potential against systemic inflammatory diseases. This study aimed to investigate the pharmacological activity and molecular mechanism of Cel in models of endotoxemia and sepsis.
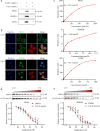
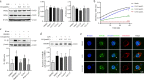
Methods: We evaluated the anti-inflammatory efficacy of Cel against endotoxemia and sepsis in mice and macrophage cultures treated with lipopolysaccharide (LPS). We screened for potential protein targets of Cel using activity-based protein profiling (ABPP). Potential targets were validated using biophysical methods such as cellular thermal shift assays (CETSA) and surface plasmon resonance (SPR). Residues involved in Cel binding to target proteins were identified through point mutagenesis, and the functional effects of such binding were explored through gene knockdown.
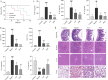
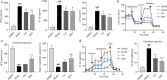
Results: Cel protected mice from lethal endotoxemia and improved their survival with sepsis, and it significantly decreased the levels of pro-inflammatory cytokines in mice and macrophages treated with LPS (P < 0.05). Cel bound to Cys424 of pyruvate kinase M2 (PKM2), inhibiting the enzyme and thereby suppressing aerobic glycolysis (Warburg effect). Cel also bound to Cys106 in high mobility group box 1 (HMGB1) protein, reducing the secretion of inflammatory cytokine interleukin (IL)-1β. Cel bound to the Cys residues in lactate dehydrogenase A (LDHA).
Conclusion: Cel inhibits inflammation and the Warburg effect in sepsis via targeting PKM2 and HMGB1 protein.
Keywords: Aerobic glycolysis; Celastrol; High mobility group box 1; Pyruvate kinase M2; Sepsis.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




Similar articles
-
Deoxyelephantopin decreases the release of inflammatory cytokines in macrophage associated with attenuation of aerobic glycolysis via modulation of PKM2.Int Immunopharmacol. 2020 Feb;79:106048. doi: 10.1016/j.intimp.2019.106048. Epub 2019 Dec 18. Int Immunopharmacol. 2020. PMID: 31863924
-
[Mechanism of WAVE1 regulation of lipopolysaccharide-induced mitochondrial metabolic abnormalities and inflammatory responses in macrophages].Zhongguo Dang Dai Er Ke Za Zhi. 2024 Dec 15;26(12):1341-1351. doi: 10.7499/j.issn.1008-8830.2408083. Zhongguo Dang Dai Er Ke Za Zhi. 2024. PMID: 39725399 Free PMC article. Chinese.
-
Melittin ameliorates inflammation in mouse acute liver failure via inhibition of PKM2-mediated Warburg effect.Acta Pharmacol Sin. 2021 Aug;42(8):1256-1266. doi: 10.1038/s41401-020-00516-0. Epub 2020 Sep 16. Acta Pharmacol Sin. 2021. PMID: 32939034 Free PMC article.
-
HMGB1, a potent proinflammatory cytokine in sepsis.Cytokine. 2010 Aug;51(2):119-26. doi: 10.1016/j.cyto.2010.02.021. Epub 2010 Mar 26. Cytokine. 2010. PMID: 20347329 Review.
-
HMGB1 as a cytokine and therapeutic target.J Endotoxin Res. 2002;8(6):469-72. doi: 10.1179/096805102125001091. J Endotoxin Res. 2002. PMID: 12697092 Review.
Cited by
-
Withaferin A and Celastrol Overwhelm Proteostasis.Int J Mol Sci. 2023 Dec 27;25(1):367. doi: 10.3390/ijms25010367. Int J Mol Sci. 2023. PMID: 38203539 Free PMC article. Review.
-
Plumbagin inhibits fungal growth, HMGB1/LOX-1 pathway and inflammatory factors in A. fumigatus keratitis.Front Microbiol. 2024 Apr 9;15:1383509. doi: 10.3389/fmicb.2024.1383509. eCollection 2024. Front Microbiol. 2024. PMID: 38655086 Free PMC article.
-
MTA1, a Novel ATP Synthase Complex Modulator, Enhances Colon Cancer Liver Metastasis by Driving Mitochondrial Metabolism Reprogramming.Adv Sci (Weinh). 2023 Sep;10(25):e2300756. doi: 10.1002/advs.202300756. Epub 2023 Jul 13. Adv Sci (Weinh). 2023. PMID: 37442756 Free PMC article.
-
Single-cell transcriptome analysis reveals the regulatory effects of artesunate on splenic immune cells in polymicrobial sepsis.J Pharm Anal. 2023 Jul;13(7):817-829. doi: 10.1016/j.jpha.2023.02.006. Epub 2023 Feb 24. J Pharm Anal. 2023. PMID: 37577384 Free PMC article.
-
Celastrol Ameliorates Neuronal Mitochondrial Dysfunction Induced by Intracerebral Hemorrhage via Targeting cAMP-Activated Exchange Protein-1.Adv Sci (Weinh). 2024 May;11(19):e2307556. doi: 10.1002/advs.202307556. Epub 2024 Mar 14. Adv Sci (Weinh). 2024. PMID: 38482725 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

