Oxidative stress facilitates infection of the unicellular alga Haematococcus pluvialis by the fungus Paraphysoderma sedebokerense
- PMID: 35596207
- PMCID: PMC9123766
- DOI: 10.1186/s13068-022-02140-y
Oxidative stress facilitates infection of the unicellular alga Haematococcus pluvialis by the fungus Paraphysoderma sedebokerense
Abstract
Background: The green microalga Haematococcus pluvialis is used as a cell factory for producing astaxanthin, the high-value carotenoid with multiple biological functions. However, H. pluvialis is prone to the infection by a parasitic fungus Paraphysoderma sedebokerense, which is the most devastating threat to the mass culture of H. pluvialis all over the world. Through dissecting the mechanisms underlying the infection process, effective measures could be developed to mitigate the pathogen threatening for the natural astaxanthin industry. By far, understanding about the interaction between the algal host and fungal pathogen remains very limited.
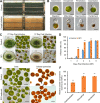
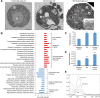
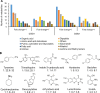
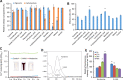
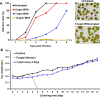
Results: We observed that there were heat-stable substances with small molecular weight produced during the infection process and enhanced the susceptibility of H. pluvialis cells to the pathogen. The infection ratio increased from 10.2% (for the algal cells treated with the BG11 medium as the control) to 52.9% (for the algal cells treated with supernatant contained such substances) on the second day post-infection, indicating the yet unknown substances in the supernatant stimulated the parasitism process. Systematic approaches including multi-omics, biochemical and imaging analysis were deployed to uncover the identity of the metabolites and the underlying mechanisms. Two metabolites, 3-hydroxyanthranilic acid and hordenine were identified and proved to stimulate the infection via driving oxidative stress to the algal cells. These metabolites generated hydroxyl radicals to disrupt the subcellular components of the algal cells and to make the algal cells more susceptible to the infection. Based on these findings, a biosafe and environment-friendly antioxidant butylated hydroxyanisole (BHA) was selected to inhibit the fungal infection, which completely abolished the infection at 12 ppm. By applying 7 ppm BHA every 2 days to the algal cell culture infected with P. sedebokerense in the 100 L open raceway ponds, the biomass of H. pluvialis reached 0.448 g/L, which was comparable to that of the control (0.473 g/L).
Conclusions: This study provides for the first time, a framework to dissect the functions of secondary metabolites in the interaction between the unicellular alga H. pluvialis and its fungal parasite, indicating that oxidative degradation is a strategy used for the fungal infest. Eliminating the oxidative burst through adding antioxidant BHA could be an effective measure to reduce parasitic infection in H. pluvialis mass culture.
Keywords: Antioxidant; Fungal pathogen; Haematococcus pluvialis; Oxidative stress; Secondary metabolites.
© 2022. The Author(s).
Conflict of interest statement
All authors declare that they have no competing interests.
Figures
Similar articles
-
Folate-mediated one-carbon metabolism as a potential antifungal target for the sustainable cultivation of microalga Haematococcus pluvialis.Biotechnol Biofuels Bioprod. 2023 Jun 17;16(1):104. doi: 10.1186/s13068-023-02353-9. Biotechnol Biofuels Bioprod. 2023. PMID: 37330505 Free PMC article.
-
Application of surfactants for controlling destructive fungus contamination in mass cultivation of Haematococcus pluvialis.Bioresour Technol. 2020 Dec;317:124025. doi: 10.1016/j.biortech.2020.124025. Epub 2020 Aug 15. Bioresour Technol. 2020. PMID: 32836037
-
Paraphysoderma sedebokerense Infection in Three Economically Valuable Microalgae: Host Preference Correlates with Parasite Fitness.J Fungi (Basel). 2021 Feb 1;7(2):100. doi: 10.3390/jof7020100. J Fungi (Basel). 2021. PMID: 33535515 Free PMC article.
-
Cell disruption and astaxanthin extraction from Haematococcus pluvialis: Recent advances.Bioresour Technol. 2022 Jan;343:126124. doi: 10.1016/j.biortech.2021.126124. Epub 2021 Oct 12. Bioresour Technol. 2022. PMID: 34653624 Review.
-
Using green alga Haematococcus pluvialis for astaxanthin and lipid co-production: Advances and outlook.Bioresour Technol. 2021 Nov;340:125736. doi: 10.1016/j.biortech.2021.125736. Epub 2021 Aug 10. Bioresour Technol. 2021. PMID: 34426245 Review.
Cited by
-
Folate-mediated one-carbon metabolism as a potential antifungal target for the sustainable cultivation of microalga Haematococcus pluvialis.Biotechnol Biofuels Bioprod. 2023 Jun 17;16(1):104. doi: 10.1186/s13068-023-02353-9. Biotechnol Biofuels Bioprod. 2023. PMID: 37330505 Free PMC article.
-
Haematococcus pluvialis culture contaminated with chytrids: growth management and astaxanthin production.Antonie Van Leeuwenhoek. 2025 Jul 15;118(8):114. doi: 10.1007/s10482-025-02129-1. Antonie Van Leeuwenhoek. 2025. PMID: 40665072
-
Importance, structure, cultivability, and resilience of the bacterial microbiota during infection of laboratory-grown Haematococcus spp. by the blastocladialean pathogen Paraphysoderma sedebokerense: evidence for a domesticated microbiota and its potential for biocontrol.FEMS Microbiol Ecol. 2025 Jan 28;101(2):fiaf011. doi: 10.1093/femsec/fiaf011. FEMS Microbiol Ecol. 2025. PMID: 39832809 Free PMC article.
References
-
- Asatryan A, Boussiba S, Zarka A. Stimulation and Isolation of Paraphysoderma sedebokerense (Blastocladiomycota) propagules and their infection capacity toward their host under different physiological and environmental conditions. Front Cell Infect Microbiol. 2019;9:72. doi: 10.3389/fcimb.2019.00072. - DOI - PMC - PubMed
-
- Borowitzka MA, Vonshak A. Scaling up microalgal cultures to commercial scale. Eur J Phycol. 2017;52(4):407–418. doi: 10.1080/09670262.2017.1365177. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
