Review article: latent tuberculosis in patients with inflammatory bowel diseases receiving immunosuppression-risks, screening, diagnosis and management
- PMID: 35596242
- PMCID: PMC9325436
- DOI: 10.1111/apt.16952
Review article: latent tuberculosis in patients with inflammatory bowel diseases receiving immunosuppression-risks, screening, diagnosis and management
Abstract
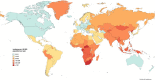
Background: One quarter of the world's population has latent tuberculosis infection (LTBI). Systemic immunosuppression is a risk factor for LTBI reactivation and the development of active tuberculosis. Such reactivation carries a risk of significant morbidity and mortality. Despite the increasing global incidence of inflammatory bowel disease (IBD) and the use of immune-based therapies, current guidelines on the testing and treatment of LTBI in patients with IBD are haphazard with a paucity of evidence.
Aim: To review the screening, diagnostic practices and medical management of LTBI in patients with IBD.
Methods: Published literature was reviewed, and recommendations for testing and treatment were synthesised by experts in both infectious diseases and IBD.
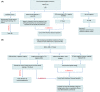
Results: Screening for LTBI should be performed proactively and includes assessment of risk factors, an interferon-gamma releasing assay or tuberculin skin test and chest X-ray. LTBI treatment in patients with IBD is scenario-dependent, related to geographical endemicity, travel and other factors. Ideally, LTBI therapy should be used prior to immune suppression but can be applied concurrently where urgent IBD medical treatment is required. Management is best directed by a multidisciplinary team involving gastroenterologists, infectious diseases specialists and pharmacists. Ongoing surveillance is recommended during therapy. Newer LTBI therapies show promise, but medication interactions need to be considered. There are major gaps in evidence, particularly with specific newer therapeutic approaches to IBD.
Conclusions: Proactive screening for LTBI is essential in patients with IBD undergoing immune-suppressing therapy and several therapeutic strategies are available. Reporting of real-world experience is essential to refining current management recommendations.
© 2022 The Authors. Alimentary Pharmacology & Therapeutics published by John Wiley & Sons Ltd.
Figures

Comment in
-
Letter: Ozanimod and latent tuberculosis.Aliment Pharmacol Ther. 2023 Feb;57(3):353-354. doi: 10.1111/apt.17311. Aliment Pharmacol Ther. 2023. PMID: 36641788 No abstract available.
References
-
- World health organisation. Global tuberculosis report. Geneva: World Health Organisation; 2021. [cited 2002 May 17]. Available from: https://www.who.int/publications/digital/global‐tuberculosis‐report‐2021...
-
- Rao M and 7 others. Latent TB infection (LTBI) ‐ mycobacterium tuberculosis pathogenesis and the dynamics of the granuloma battleground. Int J Infect Dis. 2019;80S:S58–61. - PubMed
-
- Menzies NA, Cohen T, Salomon JA. Evidence sources on the natural history of latent tuberculosis infection. Lancet Infect Dis. 2018;18(8):834–5. - PubMed
-
- Trauer JM and 6 others. Risk of active tuberculosis in the five years following infection… 15%? Chest. 2016;149(2):516–25. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
