Intravenous Perampanel as an Interchangeable Alternative to Oral Perampanel: A Randomized, Crossover, Phase I Pharmacokinetic and Safety Study
- PMID: 35596529
- PMCID: PMC9320958
- DOI: 10.1002/cpdd.1107
Intravenous Perampanel as an Interchangeable Alternative to Oral Perampanel: A Randomized, Crossover, Phase I Pharmacokinetic and Safety Study
Abstract
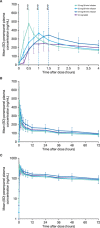
Intravenous (IV) drug administration enables treatment of epilepsy when oral administration is temporarily not feasible. Perampanel is a once-daily antiseizure medication currently available as oral formulations. Study 050 (NCT03376997) was an open-label, randomized, single-dose, crossover study to evaluate the interchangeability of oral and IV perampanel in healthy subjects (N = 48). Bioequivalence of single 12-mg doses of IV (30-, 60-, or 90-minute infusion) and oral perampanel, ≥6 weeks apart, was assessed. Analyses indicated bioequivalence of area under the plasma concentration-time curve extrapolated to infinity for 30- and 60-minute IV infusions and oral perampanel doses (geometric mean ratio [90% confidence interval], 0.93 [0.84-1.02] and 1.03 [0.97-1.09], respectively); however, IV maximum observed drug concentration (Cmax ) values were 1.35- to 1.61-fold higher than Cmax . Simulated plasma concentration-time profiles using pooled pharmacokinetic data further supported oral and IV perampanel interchangeability in two scenarios: 12-mg per day IV dosing during a temporary 7-day switch from oral steady-state maintenance therapy, and treatment initiation with 2-mg perampanel. Thirty-four (70.8%) subjects experienced treatment-related adverse events. The IV perampanel safety profile was similar to that of oral perampanel without new safety concerns. Perampanel IV infusions may be a suitable temporary alternative to oral perampanel for treatment maintenance and/or initiation.
Keywords: antiseizure medication; bioavailability/bioequivalence; epilepsy; intravenous; perampanel.
© 2022 Eisai Inc. Clinical Pharmacology in Drug Development published by Wiley Periodicals LLC on behalf of American College of Clinical Pharmacology.
Conflict of interest statement
Z.H., O.M., and P.B. are employees of Eisai Europe Ltd. J.A., L.Y.N., and L.R. are employees of Eisai Inc. Medical writing support, under the direction of the authors, was provided by Laura George, PhD, on behalf of CMC AFFINITY, McCann Health Medical Communications, funded by Eisai Inc., in accordance with Good Publication Practice (GPP3) guidelines.
Figures

References
-
- World Health Organization (WHO) . Epilepsy Key Facts. https://www.who.int/news‐room/fact‐sheets/detail/epilepsy. Accessed October 5, 2021.
-
- Harden C, Tomson T, Gloss D, et al. Practice guideline summary: sudden unexpected death in epilepsy incidence rates and risk factors: Report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology and the American Epilepsy Society. Epilepsy Curr. 2017;17(3):180‐187. - PMC - PubMed
-
- Hesdorffer DC, Tomson T, Benn E, et al. Do antiepileptic drugs or generalized tonic‐clonic seizure frequency increase SUDEP risk? A combined analysis. Epilepsia. 2012;53(2):249‐252. - PubMed
-
- Asadi‐Pooya AA, Nikseresht A, Yaghoubi E, Nei M. Physical injuries in patients with epilepsy and their associated risk factors. Seizure. 2012;21(3):165‐168. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

