NRF2 Serves a Critical Role in Regulation of Immune Checkpoint Proteins (ICPs) During Trophoblast Differentiation
- PMID: 35596653
- PMCID: PMC9197021
- DOI: 10.1210/endocr/bqac070
NRF2 Serves a Critical Role in Regulation of Immune Checkpoint Proteins (ICPs) During Trophoblast Differentiation
Abstract
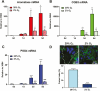
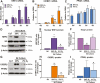
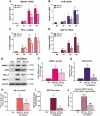
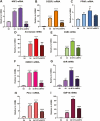
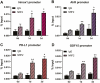
Using cultured human trophoblast stem cells (hTSCs), mid-gestation human trophoblasts in primary culture, and gene-targeted mice, we tested the hypothesis that the multinucleated syncytiotrophoblast (SynT) serves a critical role in pregnancy maintenance through production of key immune modulators/checkpoint proteins (ICPs) under control of the O2-regulated transcription factor, NRF2/NFE2L2. These ICPs potentially act at the maternal-fetal interface to protect the hemiallogeneic fetus from rejection by the maternal immune system. Using cultured hTSCs, we observed that several ICPs involved in the induction and maintenance of immune tolerance were markedly upregulated during differentiation of cytotrophoblasts (CytTs) to SynT. These included HMOX1, kynurenine receptor, aryl hydrocarbon receptor, PD-L1, and GDF15. Intriguingly, NRF2, C/EBPβ, and PPARγ were markedly induced when CytTs fused to form SynT in a 20% O2 environment. Notably, when hTSCs were cultured in a hypoxic (2% O2) environment, SynT fusion and the differentiation-associated induction of NRF2, C/EBPβ, aromatase (CYP19A1; SynT differentiation marker), and ICPs were blocked. NRF2 knockdown also prevented induction of aromatase, C/EBPβ and the previously mentioned ICPs. Chromatin immunoprecipitation-quantitative PCR revealed that temporal induction of the ICPs in hTSCs and mid-gestation human trophoblasts cultured in 20% O2 was associated with increased binding of endogenous NRF2 to putative response elements within their promoters. Moreover, placentas of 12.5 days postcoitum mice with a global Nrf2 knockout manifested decreased mRNA expression of C/ebpβ, Pparγ, Hmox1, aryl hydrocarbon receptor, and Nqo1, another direct downstream target of Nrf2, compared with wild-type mice. Collectively, these compelling findings suggest that O2-regulated NRF2 serves as a key regulator of ICP expression during SynT differentiation.
Keywords: C/EBPβ; NRF2; immune modulators/checkpoint proteins (ICPs); trophoblast.
© The Author(s) 2022. Published by Oxford University Press on behalf of the Endocrine Society. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Figures


Comment in
-
Revealing Molecular Mechanisms Supporting Trophoblast-Mediated Maternal Immune Tolerance.Endocrinology. 2022 Aug 1;163(8):bqac099. doi: 10.1210/endocr/bqac099. Endocrinology. 2022. PMID: 35797586 No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

