Combination of docetaxel versus nonsteroidal antiandrogen with androgen deprivation therapy for high-volume metastatic hormone-sensitive prostate cancer: a propensity score-matched analysis
- PMID: 35596809
- PMCID: PMC10415473
- DOI: 10.1007/s00345-022-04030-2
Combination of docetaxel versus nonsteroidal antiandrogen with androgen deprivation therapy for high-volume metastatic hormone-sensitive prostate cancer: a propensity score-matched analysis
Abstract
Purpose: The aim of this study was to investigate the oncologic efficacy of combining docetaxel with androgen deprivation therapy (ADT) versus nonsteroidal antiandrogen (NSAA) with ADT in patients with high-volume metastatic hormone-sensitive prostate cancer (mHSPC) with focus on the effect of sequential therapy in a real-world clinical practice setting.
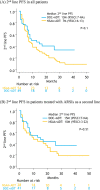
Methods: The records of 382 patients who harbored high-volume mHSPC, based on the CHAARTED criteria, and had received ADT with either docetaxel (n = 92) or NSAA (bicalutamide) (n = 290) were retrospectively analyzed. The cohorts were matched by one-to-one propensity scores based on patient demographics. Overall survival (OS), cancer-specific survival (CSS), progression-free survival (PFS), including time to castration-resistant prostate cancer (CRPC), and time to second-line progression (PFS2) were compared. 2nd-line PFS defined as the time from CRPC diagnosis to progression after second-line therapy was also compared.
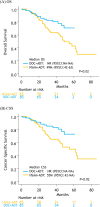
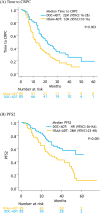
Results: After matching, a total of 170 patients were retained: 85 patients treated with docetaxel + ADT and 85 patients treated with NSAA + ADT. The median OS and CSS for docetaxel + ADT versus NSAA + ADT were not reached (NR) vs. 49 months (p = 0.02) and NR vs. 55 months (p = 0.02), respectively. Median time to CRPC and PFS2 in patients treated with docetaxel + ADT was significantly longer compared to those treated with NSAA (22 vs. 12 months; p = 0.003 and, NR vs. 28 months; p < 0.001, respectively). There was no significant difference in 2nd-line PFS between the two groups.
Conclusions: Our analysis suggested that ADT with docetaxel significantly prolonged OS and CSS owing to a better time to CRPC and PFS2 in comparison to NSAA + ADT in high-volume mHSPC.
Keywords: Bicalutamide; Docetaxel; High volume; Metastatic hormone-sensitive prostate cancer; Nonsteroidal antiandrogen.
© 2022. The Author(s).
Conflict of interest statement
・Takahiro Kimura is a paid consultant/advisor of Astellas, Bayer, Janssen, and Sanofi. Shintaro Narita received honoraria from Janssen Pharmaceutical K.K., Bayer AG. Dr., AstraZeneca K.K., Takeda Pharmaceutical Company Ltd., Sanofi S.A., Astellas Pharma Inc., and grants for research from Novartis Pharmaceuticals. Shingo Hatakeyama received honoraria from Janssen Pharmaceutical K.K. and Pfizer Inc. Department of Advanced Blood Purification Therapy is an endowment department, supported by a grant from NIPRO Corporation. Tomonori Habuchi received honoraria from Janssen Pharmaceutical K.K., Takeda Pharmaceutical Company Ltd., Astellas Pharma Inc., Daiichi Sankyo Company, Ltd., AstraZeneca K.K., Sanofi S.A., and Bayer AG. Chikara Ohyama received honoraria from Astellas Pharma Inc., NIPPON SHINYAKU Company Ltd., AstraZeneca K.K., Janssen Pharmaceutical K.K., Takeda Pharmaceutical Company Ltd., Novartis Pharma K.K., ONO Pharmaceutical Company Ltd., Chugai Pharmaceutical Company Ltd., Sanofi S.A., Bayer AG., Pfizer Inc., Bristol Myers Squibb, Otsuka Pharmaceutical Company Ltd., KISSEI Pharmaceutical Company Ltd., Kyowa Kirin Company Ltd., Daiichi Sankyo Company Ltd., KANEKA Corporation, and Nipro Corporation. Shahrokh F. Shariat received as follows: Honoraria: Astellas, AstraZeneca, BMS, Ferring, Ipsen, Janssen, MSD, Olympus, Pfizer, Roche, and Takeda Consulting or Advisory Role: Astellas, AstraZeneca, BMS, Ferring, Ipsen, Janssen, MSD, Olympus, Pfizer, Pierre Fabre, Roche, and Takeda Speakers Bureau: Astellas, Astra Zeneca, Bayer, BMS, Ferring, Ipsen, Janssen, MSD, Olympus, Pfizer, Richard Wolf, Roche, and Takeda Shin Egawa is a paid consultant/advisor of Takeda, Astellas, AstraZeneca, Sanofi, Janssen, and Pfizer. The other authors declare no conflicts of interest associated with this manuscript.
Figures
References
-
- James ND, Sydes MR, Clarke NW, Mason MD, Dearnaley DP, Spears MR, et al. Addition of docetaxel, zoledronic acid, or both to first-line long-term hormone therapy in prostate cancer (STAMPEDE): survival results from an adaptive, multiarm, multistage, platform randomised controlled trial. Lancet. 2016;387(10024):1163–77. doi: 10.1016/S0140-6736(15)01037-5. - DOI - PMC - PubMed
-
- Cornford P, van den Bergh RCN, Briers E, Van den Broeck T, Cumberbatch MG, De Santis M, et al. EAU-EANM-ESTRO-ESUR-SIOG guidelines on prostate cancer. Part II-2020 update: treatment of relapsing and metastatic prostate cancer. Eur Urol. 2021;79(2):263–82. doi: 10.1016/j.eururo.2020.09.046. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

