Current and Emerging Strategies to Inhibit Type 2 Inflammation in Atopic Dermatitis
- PMID: 35596901
- PMCID: PMC9276864
- DOI: 10.1007/s13555-022-00737-7
Current and Emerging Strategies to Inhibit Type 2 Inflammation in Atopic Dermatitis
Abstract
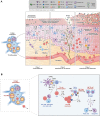
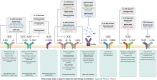
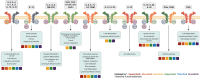
Type 2 immunity evolved to combat helminth infections by orchestrating a combined protective response of innate and adaptive immune cells and promotion of parasitic worm destruction or expulsion, wound repair, and barrier function. Aberrant type 2 immune responses are associated with allergic conditions characterized by chronic tissue inflammation, including atopic dermatitis (AD) and asthma. Signature cytokines of type 2 immunity include interleukin (IL)-4, IL-5, IL-9, IL-13, and IL-31, mainly secreted from immune cells, as well as IL-25, IL-33, and thymic stromal lymphopoietin, mainly secreted from tissue cells, particularly epithelial cells. IL-4 and IL-13 are key players mediating the prototypical type 2 response; IL-4 initiates and promotes differentiation and proliferation of naïve T-helper (Th) cells toward a Th2 cell phenotype, whereas IL-13 has a pleiotropic effect on type 2 inflammation, including, together with IL-4, decreased barrier function. Both cytokines are implicated in B-cell isotype class switching to generate immunoglobulin E, tissue fibrosis, and pruritus. IL-5, a key regulator of eosinophils, is responsible for eosinophil growth, differentiation, survival, and mobilization. In AD, IL-4, IL-13, and IL-31 are associated with sensory nerve sensitization and itch, leading to scratching that further exacerbates inflammation and barrier dysfunction. Various strategies have emerged to suppress type 2 inflammation, including biologics targeting cytokines or their receptors, and Janus kinase inhibitors that block intracellular cytokine signaling pathways. Here we review type 2 inflammation, its role in inflammatory diseases, and current and future therapies targeting type 2 pathways, with a focus on AD. INFOGRAPHIC.
Keywords: Atopic dermatitis; Biologic; Cytokine; Janus kinase; Type 2 inflammation.
© 2022. The Author(s).
Figures

References
-
- Maizels RM. Regulation of immunity and allergy by helminth parasites. Allergy. 2020;75(3):524–534. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

