Assessing chemotherapy-induced peripheral neuropathy with patient reported outcome measures: a systematic review of measurement properties and considerations for future use
- PMID: 35596913
- PMCID: PMC9546984
- DOI: 10.1007/s11136-022-03154-7
Assessing chemotherapy-induced peripheral neuropathy with patient reported outcome measures: a systematic review of measurement properties and considerations for future use
Abstract
Purpose: Chemotherapy-induced peripheral neuropathy (CIPN) is a common toxicity of cancer treatment, with potential to significantly impact cancer survivors' long-term quality of life. Patient reported outcome measures (PROMs) are increasingly utilised to evaluate CIPN. However, guidance remains lacking on how to identify fit for purpose PROMs with considerations necessarily differing when used in various research and in-clinic contexts. This study aimed to evaluate evidence about CIPN PROMs measurement properties and propose considerations to optimize CIPN PROM selection for each purpose.
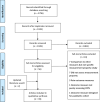
Methods: A systematic review was conducted to identify literature assessing measurement properties of CIPN PROMs. These were evaluated against Consensus-based Standards for the selection of health Measurement Instruments (COSMIN) criteria and International Society for Quality of Life minimum standards. Risk of Bias (RoB) was assessed using the COSMIN RoB checklist.
Results: Thirty-nine papers evaluating measurement properties of 13 PROMs were included. The European Organization for Research and Treatment of Cancer Quality of Life Chemotherapy-Induced Peripheral Neuropathy Questionnaire (QLQ-CIPN20) and Functional Assessment of Cancer Therapy/Gynecologic Oncology Group-Neurotoxicity (FACT/GOG-Ntx) were the most commonly investigated PROMs and had the most measurement properties meeting established criteria.
Conclusion: The use of the QLQ-CIPN20 and FACT/GOG-Ntx to assess CIPN in research settings has the most supporting evidence. However other considerations including study aims, endpoints and target population also factor into PROM selection and need to be considered more often when determining the most suitable outcome measure. Evidence of CIPN PROMs use in clinical practice is limited and their adoption to individual-patient level management requires more evaluation.
Keywords: Cancer survivors; Chemotherapy-induced peripheral neuropathy; Measurement properties; Patient reported outcome measures; Psychometric evaluation; Systematic review.
© 2022. The Author(s).
Conflict of interest statement
The authors have no conflict of interest to declare.
Figures
References
-
- Loprinzi CL, Lacchetti C, Bleeker J, Cavaletti G, Chauhan C, Hertz DL, Kelley MR, Lavino A, Lustberg MB, Paice JA, Schneider BP, Lavoie Smith EM, Smith ML, Smith TJ, Wagner-Johnston N, Hershman DL. Prevention and management of chemotherapy-induced peripheral neuropathy in survivors of adult cancers: ASCO guideline update. Journal of Clinical Oncology. 2020;38(28):3325–3348. doi: 10.1200/jco.20.01399. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- 14/TPG/1-05/Cancer Institute NSW
- Project Grant #1080521/National Health and Medical Research Council
- Career Development Fellowship #1148595/National Health and Medical Research Council
- Program Grant #1132524/National Health and Medical Research Council
- Partnership Project #1153439/National Health and Medical Research Council
LinkOut - more resources
Full Text Sources
Medical