The new era of add-on asthma treatments: where do we stand?
- PMID: 35598022
- PMCID: PMC9124422
- DOI: 10.1186/s13223-022-00676-0
The new era of add-on asthma treatments: where do we stand?
Abstract
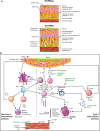
Globally, a small proportion (5-12%) of asthma patients are estimated to have severe disease. However, severe asthma accounts for disproportionately high healthcare resource utilization. The Global Initiative for Asthma (GINA) management committee recommends treating patients with asthma with inhaled corticosteroids plus long-acting β2-agonists and, when needed, adding a long-acting muscarinic receptor antagonist or biologic agent. Five biologics, targeting different effectors in the type 2 inflammatory pathway, are approved for asthma treatment. However, biologics have not been compared against each other or add-on inhaled therapies in head-to-head clinical trials. As a result, their positioning versus that of current and anticipated small-molecule strategies is largely unknown. Furthermore, with the emergence of biomarkers for predicting response to biologics, a more personalized treatment approach-currently lacking with inhaled therapies-may be possible. To gain perspective, we reviewed recent advances in asthma pathophysiology, phenotypes, and biomarkers; the place of biologics in the management and personalized treatment of severe asthma; and the future of biologics and small-molecule drugs. We propose an algorithm for the stepwise treatment of severe asthma based on recommendations in the GINA strategy document that accounts for the broad range of phenotypes targeted by inhaled therapies and the specificity of biologics. In the future, both biologics and small molecules will continue to play key roles in the individualized treatment of severe asthma. However, as targeted therapies, their application will continue to be focused on patients with certain phenotypes who meet the specific criteria for use as identified in pivotal clinical trials.
Keywords: Add-on; Add-on therapy; Biological therapy; Severe asthma; Tiotropium bromide.
© 2022. The Author(s).
Conflict of interest statement
GLC reports personal fees and other fees (consultant, speakers bureau, and clinical trial site) from GlaxoSmithKline, Boehringer Ingelheim Pharmaceuticals, Genentech, AstraZeneca, Sanofi Genzyme, and Regeneron outside the submitted work. WJC report personal fees from Genentech and grants from AstraZeneca and Sanofi, outside the submitted work.
Figures


References
-
- Global Initiative for Asthma. Global strategy for asthma management and prevention; 2021. https://ginasthma.org/gina-reports/. Accessed 10 June 2021.
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

