Unsupervised brain imaging 3D anomaly detection and segmentation with transformers
- PMID: 35598520
- PMCID: PMC10108352
- DOI: 10.1016/j.media.2022.102475
Unsupervised brain imaging 3D anomaly detection and segmentation with transformers
Abstract
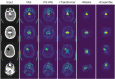
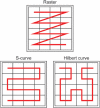
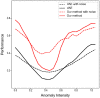
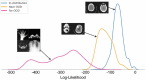
Pathological brain appearances may be so heterogeneous as to be intelligible only as anomalies, defined by their deviation from normality rather than any specific set of pathological features. Amongst the hardest tasks in medical imaging, detecting such anomalies requires models of the normal brain that combine compactness with the expressivity of the complex, long-range interactions that characterise its structural organisation. These are requirements transformers have arguably greater potential to satisfy than other current candidate architectures, but their application has been inhibited by their demands on data and computational resources. Here we combine the latent representation of vector quantised variational autoencoders with an ensemble of autoregressive transformers to enable unsupervised anomaly detection and segmentation defined by deviation from healthy brain imaging data, achievable at low computational cost, within relative modest data regimes. We compare our method to current state-of-the-art approaches across a series of experiments with 2D and 3D data involving synthetic and real pathological lesions. On real lesions, we train our models on 15,000 radiologically normal participants from UK Biobank and evaluate performance on four different brain MR datasets with small vessel disease, demyelinating lesions, and tumours. We demonstrate superior anomaly detection performance both image-wise and pixel/voxel-wise, achievable without post-processing. These results draw attention to the potential of transformers in this most challenging of imaging tasks.
Keywords: Anomaly detection; Transformer; Unsupervised anomaly segmentation; Vector quantized variational autoencoder.
Copyright © 2022. Published by Elsevier B.V.
Conflict of interest statement
Declaration of Competing Interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures

Similar articles
-
Cross Attention Transformers for Multi-modal Unsupervised Whole-Body PET Anomaly Detection.Deep Gener Model (2022). 2022;13609:14-23. doi: 10.1007/978-3-031-18576-2_2. Epub 2022 Oct 8. Deep Gener Model (2022). 2022. PMID: 39404690 Free PMC article.
-
Self-Supervised Anomaly Detection from Anomalous Training Data via Iterative Latent Token Masking.IEEE Int Conf Comput Vis Workshops. 2023 Dec 25;2023:2394-2402. doi: 10.1109/ICCVW60793.2023.00254. IEEE Int Conf Comput Vis Workshops. 2023. PMID: 39205863 Free PMC article.
-
Three-dimensional deep learning with spatial erasing for unsupervised anomaly segmentation in brain MRI.Int J Comput Assist Radiol Surg. 2021 Sep;16(9):1413-1423. doi: 10.1007/s11548-021-02451-9. Epub 2021 Jul 12. Int J Comput Assist Radiol Surg. 2021. PMID: 34251654 Free PMC article.
-
Autoencoders for unsupervised anomaly segmentation in brain MR images: A comparative study.Med Image Anal. 2021 Apr;69:101952. doi: 10.1016/j.media.2020.101952. Epub 2021 Jan 2. Med Image Anal. 2021. PMID: 33454602 Review.
-
Do it the transformer way: A comprehensive review of brain and vision transformers for autism spectrum disorder diagnosis and classification.Comput Biol Med. 2023 Dec;167:107667. doi: 10.1016/j.compbiomed.2023.107667. Epub 2023 Nov 3. Comput Biol Med. 2023. PMID: 37939407 Review.
Cited by
-
Evaluating the use of synthetic T1-w images in new T2 lesion detection in multiple sclerosis.Front Neurosci. 2022 Sep 29;16:954662. doi: 10.3389/fnins.2022.954662. eCollection 2022. Front Neurosci. 2022. PMID: 36248650 Free PMC article.
-
Geometry-invariant abnormality detection.Med Image Comput Comput Assist Interv. 2023 Jan 10;2023:300-309. doi: 10.1007/978-3-031-43907-0_29. Med Image Comput Comput Assist Interv. 2023. PMID: 39206415 Free PMC article.
-
Cross Attention Transformers for Multi-modal Unsupervised Whole-Body PET Anomaly Detection.Deep Gener Model (2022). 2022;13609:14-23. doi: 10.1007/978-3-031-18576-2_2. Epub 2022 Oct 8. Deep Gener Model (2022). 2022. PMID: 39404690 Free PMC article.
-
Self-Supervised Anomaly Detection from Anomalous Training Data via Iterative Latent Token Masking.IEEE Int Conf Comput Vis Workshops. 2023 Dec 25;2023:2394-2402. doi: 10.1109/ICCVW60793.2023.00254. IEEE Int Conf Comput Vis Workshops. 2023. PMID: 39205863 Free PMC article.
-
Semi-supervised Label Generation for 3D Multi-modal MRI Bone Tumor Segmentation.J Imaging Inform Med. 2025 Feb 20. doi: 10.1007/s10278-025-01448-z. Online ahead of print. J Imaging Inform Med. 2025. PMID: 39979760
References
-
- Bakas, S., Reyes, M., Jakab, A., Bauer, S., Rempfler, M., Crimi, A., Shinohara, R.T., Berger, C., Ha, S.M., Rozycki, M., 2018. Identifying the best machine learning algorithms for brain tumor segmentation, progression assessment, and overall survival prediction in the BRATS challenge. arXiv Prepr. arXiv:1811.02629.
-
- Baur, C., Denner, S., Wiestler, B., Albarqouni, S., Navab, N., 2020a. Autoencoders for Unsupervised Anomaly Segmentation in Brain MR Images: A Comparative Study. arXiv Prepr. arXiv:2004.03271. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

