Layers 3 and 4 Neurons of the Bilateral Whisker-Barrel Cortex
- PMID: 35598701
- PMCID: PMC9884091
- DOI: 10.1016/j.neuroscience.2022.05.018
Layers 3 and 4 Neurons of the Bilateral Whisker-Barrel Cortex
Abstract
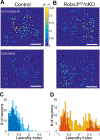
In Robo3R3-5cKO mouse brain, rhombomere 3-derived trigeminal principal nucleus (PrV) neurons project bilaterally to the somatosensory thalamus. As a consequence, whisker-specific neural modules (barreloids and barrels) representing whiskers on both sides of the face develop in the sensory thalamus and the primary somatosensory cortex. We examined the morphological complexity of layer 4 barrel cells, their postsynaptic partners in layer 3, and functional specificity of layer 3 pyramidal cells. Layer 4 spiny stellate cells form much smaller barrels and their dendritic fields are more focalized and less complex compared to controls, while layer 3 pyramidal cells did not show notable differences. Using in vivo 2-photon imaging of a genetically encoded fluorescent [Ca2+] sensor, we visualized neural activity in the normal and Robo3R3-5cKO barrel cortex in response to ipsi- and contralateral single whisker stimulation. Layer 3 neurons in control animals responded only to their contralateral whiskers, while in the mutant cortex layer 3 pyramidal neurons showed both ipsi- and contralateral whisker responses. These results indicate that bilateral whisker map inputs stimulate different but neighboring groups of layer 3 neurons which normally relay contralateral whisker-specific information to other cortical areas.
Keywords: Ca(2+)-fluorescence protein; In vivo 2-photon imaging; conditional Robo3 knockout; dendritic complexity; somatosensory cortex; whiskers.
Copyright © 2022 IBRO. All rights reserved.
Figures




References
-
- Chédotal A (2014) Development and plasticity of commissural circuits: from locomotion to brain repair. Trends Neurosci 37(10):551–62. - PubMed
-
- Da Silva RV, Johannssen H, Wyss ,MT, Roome RB, Bourojeni FB, Stifani N, Marsh APL, Ryan MM, et al. (2018) DCC is required for the development of nociceptive topognosis in Mice and Humans. Cell Rep 22(5): 1105–14. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

