Biological effects of crude oil vapor. IV. Cardiovascular effects
- PMID: 35598716
- PMCID: PMC9904414
- DOI: 10.1016/j.taap.2022.116071
Biological effects of crude oil vapor. IV. Cardiovascular effects
Abstract
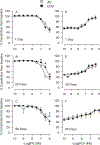
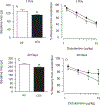
Workers in the oil and gas extraction industry are at risk of inhaling volatile organic compounds. Epidemiological studies suggest oil vapor inhalation may affect cardiovascular health. Thus, in this hazard identification study we investigated the effects of inhalation of crude oil vapor (COV) on cardiovascular function. Male rats were exposed to air or COV (300 ppm) for 6 h (acute), or 6 h/day × 4 d/wk. × 4 wk. (sub-chronic). The effects of COV inhalation were assessed 1, 28, and 90 d post-exposure. Acute exposure to COV resulted in reductions in mean arterial and diastolic blood pressures 1 and 28 d after exposure, changes in nitrate-nitrite and H2O2 levels, and in the expression of transcripts and proteins that regulate inflammation, vascular remodeling, and the synthesis of nitric oxide (NO) in the heart and kidneys. The sub-chronic exposure resulted in a reduced sensitivity to α1-adrenoreceptor-mediated vasoconstriction in vitro 28 d post-exposure, and a reduction in oxidative stress in the heart. Sub-chronic COV exposure led to alterations in the expression of NO synthases and anti-oxidant enzymes, which regulate inflammation and oxidative stress in the heart and kidneys. There seems to be a balance between changes in the expression of transcripts associated with the generation of reactive oxygen species (ROS) and antioxidant enzymes. The ability of antioxidant enzymes to reduce or inhibit the effects of ROS may allow the cardiovascular system to adapt to acute COV exposures. However, sub-chronic exposures may result in longer-lasting negative health consequences on the cardiovascular system.
Keywords: Adrenoreceptor modulation; Cardiovascular; Inflammation; Oxidative stress; Peripheral vascular; Renal.
Published by Elsevier Inc.
Conflict of interest statement
Declaration of Competing Interest
The authors declare that they have no conflicts of interest in relation to this publication.
Figures

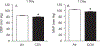

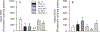


References
-
- Aguilera F, Mendez J, Pasaro E, Laffon B, 2010. Review on the effects of exposure to spilled oils on human health. J. Appl. Toxicol 30 (4), 291–301. - PubMed
-
- American Petroleum Institute, 2008. The Relationship between the Aromatic Ring Class Content and Selected Endpoints of Repeat-Dose and Developmental Toxicity of High-Boiling Petroleum Substances. cited Available from. http://www.petroleumhpv.org/~/media/PetroleumHPV/Documents/Publications/....
-
- Anttila A, Pokhrel A, Heikkila P, Viinanen R, Pukkala E, 2015. Kidney cancer risk in oil refining in Finland: a nested case-referent study. J. Occup. Environ. Med 57 (1), 68–72. - PubMed
-
- Aragon M, Erdely A, Bishop L, Salmen R, Weaver J, Liu J, Hall P, Eye T, Kodali V, Zeidler-Erdely P, Stafflinger JE, Ottens AK, Campen MJ, 2016. MMP-9-dependent serum-borne bioactivity caused by multiwalled carbon nanotube exposure induces vascular dysfunction via the CD36 scavenger receptor. Toxicol. Sci 150 (2), 488–498. - PMC - PubMed
-
- Bicu M, Mota M, Panduru NM, Graunteanu C, Mota E, 2010. Oxidative stress in diabetic kidney disease. Rom. J. Intern. Med 48 (4), 307–312. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

