Remdesivir for the treatment of COVID-19: a systematic review and meta-analysis
- PMID: 35598856
- PMCID: PMC9117160
- DOI: 10.1016/j.cmi.2022.04.018
Remdesivir for the treatment of COVID-19: a systematic review and meta-analysis
Abstract
Background: The benefits of remdesivir in the treatment of hospitalized patients with COVID-19 remain debated with the National Institutes of Health and the World Health Organization providing contradictory recommendations for and against use.
Objectives: To evaluate the role of remdesivir for hospitalized inpatients as a function of oxygen requirements.
Data sources: Beginning with our prior systematic review, we searched MEDLINE using PubMed from 15 January 2021 through 5 May 2022.
Study eligibility criteria: Randomised controlled trials; all languages.
Participants: All hospitalized adults with COVID-19.
Interventions: Remdesivir, in comparison to either placebo, or standard of care.
Assessment of risk of bias: We used the ROB-2 criteria.
Methods of data synthesis: The primary outcome was mortality, stratified by oxygen use (none, supplemental oxygen without mechanical ventilation, and mechanical ventilation). We conducted a frequentist random effects meta-analysis on the risk ratio scale and, to contextualize the probabilistic benefits, we also performed a Bayesian random effects meta-analysis on the risk difference scale. A ≥1% absolute risk reduction was considered clinically important.
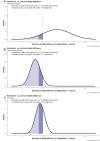
Results: We identified eight randomized trials, totaling 10 751 participants. The risk ratio for mortality comparing remdesivir vs. control was 0.77 (95% CI, 0.5-1.19) in the patients who did not require supplemental oxygen; 0.89 (95% CI, 0.79-0.99) for nonventilated patients requiring oxygen; and 1.08 (95% CI, 0.88-1.31) in the setting of mechanical ventilation. Using neutral priors, the probabilities that remdesivir reduces mortality were 76.8%, 93.8%, and 14.7%, respectively. The probability that remdesivir reduced mortality by ≥ 1% was 77.4% for nonventilated patients requiring oxygen.
Conclusions: Based on this meta-analysis, there is a high probability that remdesivir reduces mortality for nonventilated patients with COVID-19 requiring supplemental oxygen therapy. Treatment guidelines should be re-evaluated.
Keywords: COVID-19; Coronavirus; Meta-analysis; Mortality; Remdesivir.
Copyright © 2022 European Society of Clinical Microbiology and Infectious Diseases. Published by Elsevier Ltd. All rights reserved.
Figures

Comment in
-
Re: Remdesivir for the treatment of COVID-19 by Lee et al.Clin Microbiol Infect. 2023 Jan;29(1):114. doi: 10.1016/j.cmi.2022.09.015. Epub 2022 Oct 4. Clin Microbiol Infect. 2023. PMID: 36206865 Free PMC article. No abstract available.
References
-
- Kaka A.S., MacDonald R., Linskens E.J., Langsetmo L., Vela K., Duan-Porter W., et al. Major update 2: remdesivir for adults with COVID-19: a living systematic review and meta-analysis for the American College of Physicians Practice Points. Ann Intern Med. 2022;175:701–709. doi: 10.7326/M21-4784. - DOI - PMC - PubMed
-
- Covid-19 Treatment Guidelines Panel Coronavirus disease . National Institutes of Health; 2019. (COVID-19) treatment guidelines.https://www.covid19treatmentguidelines.nih.gov/therapeutic-management/ c2021. Available at. - PubMed
-
- Bhimraj A., Morgan R., Hirsch Shumaker A., Lavergne V., Baden L., Chi-Chung Cheng V., et al. Infectious Diseases Society of America guidelines on the treatment and management of patients with COVID-19. 2022. https://www.idsociety.org/practice-guideline/covid-19-guideline-treatmen... Version 5.5.2 Available at. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

