Multiple metabolic pathways fuel the truncated tricarboxylic acid cycle of the prostate to sustain constant citrate production and secretion
- PMID: 35598879
- PMCID: PMC9168698
- DOI: 10.1016/j.molmet.2022.101516
Multiple metabolic pathways fuel the truncated tricarboxylic acid cycle of the prostate to sustain constant citrate production and secretion
Abstract
Objective: The prostate is metabolically unique: it produces high levels of citrate for secretion via a truncated tricarboxylic acid (TCA) cycle to maintain male fertility. In prostate cancer (PCa), this phenotype is reprogrammed, making it an interesting therapeutic target. However, how the truncated prostate TCA cycle works is still not completely understood.
Methods: We optimized targeted metabolomics in mouse and human organoid models in ex vivo primary culture. We then used stable isotope tracer analyses to identify the pathways that fuel citrate synthesis.
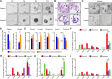
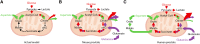
Results: First, mouse and human organoids were shown to recapitulate the unique citrate-secretory program of the prostate, thus representing a novel model that reproduces this unusual metabolic profile. Using stable isotope tracer analysis, several key nutrients were shown to allow the completion of the prostate TCA cycle, revealing a much more complex metabolic profile than originally anticipated. Indeed, along with the known pathway of aspartate replenishing oxaloacetate, glutamine was shown to fuel citrate synthesis through both glutaminolysis and reductive carboxylation in a GLS1-dependent manner. In human organoids, aspartate entered the TCA cycle at the malate entry point, upstream of oxaloacetate. Our results demonstrate that the citrate-secretory phenotype of prostate organoids is supported by the known aspartate-oxaloacetate-citrate pathway, but also by at least three additional pathways: glutaminolysis, reductive carboxylation, and aspartate-malate conversion.
Conclusions: Our results add a significant new dimension to the prostate citrate-secretory phenotype, with at least four distinct pathways being involved in citrate synthesis. Better understanding this distinctive citrate metabolic program will have applications in both male fertility as well as in the development of novel targeted anti-metabolic therapies for PCa.
Keywords: Androgen; Fertility; Organoids; Prostate cancer; TCA cycle.
Copyright © 2022 The Author(s). Published by Elsevier GmbH.. All rights reserved.
Figures



Similar articles
-
Substrate usage determines carbon flux via the citrate cycle in Helicobacter pylori.Mol Microbiol. 2021 Sep;116(3):841-860. doi: 10.1111/mmi.14775. Epub 2021 Jul 8. Mol Microbiol. 2021. PMID: 34164854
-
The redistribution of carbon label by the reactions involved in glycolysis, gluconeogenesis and the tricarboxylic acid cycle in rat liver.Biochem J. 1968 Nov;110(2):313-35. doi: 10.1042/bj1100313. Biochem J. 1968. PMID: 5726211 Free PMC article.
-
Determiners of cell fates: the tricarboxylic acid cycle versus the citrate-malate shuttle.Protein Cell. 2023 Apr 13;14(3):162-164. doi: 10.1093/procel/pwac026. Protein Cell. 2023. PMID: 37051670 Free PMC article. No abstract available.
-
Metabolic and mind shifts: from glucose to glutamine and acetate addictions in cancer.Curr Opin Clin Nutr Metab Care. 2015 Jul;18(4):346-53. doi: 10.1097/MCO.0000000000000178. Curr Opin Clin Nutr Metab Care. 2015. PMID: 26001655 Review.
-
Concepts of citrate production and secretion by prostate. 1. Metabolic relationships.Prostate. 1991;18(1):25-46. doi: 10.1002/pros.2990180104. Prostate. 1991. PMID: 1987578 Review.
Cited by
-
Mediation effect of plasma metabolites on the relationship between immune cells and the risk of prostatitis: A study by bidirectional 2-sample and Bayesian-weighted Mendelian randomization.Medicine (Baltimore). 2024 Oct 11;103(41):e40024. doi: 10.1097/MD.0000000000040024. Medicine (Baltimore). 2024. PMID: 39465812 Free PMC article.
-
Isocitrate dehydrogenase 1 sustains a hybrid cytoplasmic-mitochondrial tricarboxylic acid cycle that can be targeted for therapeutic purposes in prostate cancer.Mol Oncol. 2023 Oct;17(10):2109-2125. doi: 10.1002/1878-0261.13441. Epub 2023 Jul 19. Mol Oncol. 2023. PMID: 37086156 Free PMC article.
-
Rediscovering citrate as a biomarker for prostate cancer.Nat Rev Urol. 2024 Oct;21(10):573-575. doi: 10.1038/s41585-024-00899-3. Nat Rev Urol. 2024. PMID: 38811764 No abstract available.
-
Concentric Hybrid Nanoelectrospray Ionization-Atmospheric Pressure Chemical Ionization Source for High-Coverage Mass Spectrometry Analysis of Single-Cell Metabolomics.Adv Sci (Weinh). 2024 Apr;11(16):e2306659. doi: 10.1002/advs.202306659. Epub 2024 Feb 15. Adv Sci (Weinh). 2024. PMID: 38359005 Free PMC article.
-
Metabolic Adjustments following Glutaminase Inhibition by CB-839 in Glioblastoma Cell Lines.Cancers (Basel). 2023 Jan 15;15(2):531. doi: 10.3390/cancers15020531. Cancers (Basel). 2023. PMID: 36672480 Free PMC article.
References
-
- Costello L.C., Franklin R.B. Decreased zinc in the development and progression of malignancy: an important common relationship and potential for prevention and treatment of carcinomas. Expert Opinion on Therapeutic Targets. Jan 2017;21(1):51–66. doi: 10.1080/14728222.2017.1265506. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

