In vitro evaluation of fenfluramine and norfenfluramine as victims of drug interactions
- PMID: 35599345
- PMCID: PMC9124820
- DOI: 10.1002/prp2.958
In vitro evaluation of fenfluramine and norfenfluramine as victims of drug interactions
Abstract
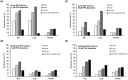
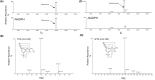
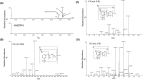
Fenfluramine (FFA) has potent antiseizure activity in severe, pharmacoresistant childhood-onset developmental and epileptic encephalopathies (e.g., Dravet syndrome). To assess risk of drug interaction affecting pharmacokinetics of FFA and its major metabolite, norfenfluramine (nFFA), we conducted in vitro metabolite characterization, reaction phenotyping, and drug transporter-mediated cellular uptake studies. FFA showed low in vitro clearance in human liver S9 fractions and in intestinal S9 fractions in all three species tested (t1/2 > 120 min). Two metabolites (nFFA and an N-oxide or a hydroxylamine) were detected in human liver microsomes versus six in dog and seven in rat liver microsomes; no metabolite was unique to humans. Selective CYP inhibitor studies showed FFA metabolism partially inhibited by quinidine (CYP2D6, 48%), phencyclidine (CYP2B6, 42%), and furafylline (CYP1A2, 32%) and, to a lesser extent (<15%), by tienilic acid (CYP2C9), esomeprazole (CYP2C19), and troleandomycin (CYP3A4/5). Incubation of nFFA with rCYP1A2, rCYP2B6, rCYP2C19, and rCYP2D6 resulted in 10%-20% metabolism and no clear inhibition of nFFA metabolism by any CYP-selective inhibitor. Reaction phenotyping showed metabolism of FFA by recombinant human cytochrome P450 (rCYP) enzymes rCYP2B6 (10%-21% disappearance for 1 and 10 µM FFA, respectively), rCYP1A2 (22%-23%), rCYP2C19 (49%-50%), and rCYP2D6 (59%-97%). Neither FFA nor nFFA was a drug transporter substrate. Results show FFA metabolism to nFFA occurs through multiple pathways of elimination. FFA dose adjustments may be needed when administered with strong inhibitors or inducers of multiple enzymes involved in FFA metabolism (e.g., stiripentol).
Keywords: antiepileptics; cytochrome P450; drug transport; drug-drug interactions.
© 2022 Zogenix, Inc. Pharmacology Research & Perspectives published by John Wiley & Sons Ltd, British Pharmacological Society and American Society for Pharmacology and Experimental Therapeutics.
Figures





References
-
- Andrade R, Barnes NM, Baxter G, et al. Hydroxytryptamine receptors (version 2019.4) in the IUPHAR/BPS guide to pharmacology database. IUPHAR/BPS Guide Pharmacol CITE. 2019;2019(4):1–31.
-
- Devinsky O, Verducci C, Thiele EA, et al. Open‐label use of highly purified CBD (Epidiolex®) in patients with CDKL5 deficiency disorder and Aicardi, Dup15q, and Doose syndromes. Epilepsy Behav. 2018;86:131‐137. - PubMed
-
- Lagae L, Schoonjans AS, Gammaitoni AR, Galer BS, Ceulemans B. A pilot, open‐label study of the effectiveness and tolerability of low‐dose ZX008 (fenfluramine HCl) in Lennox‐Gastaut syndrome. Epilepsia. 2018;59:1881‐1888. - PubMed
-
- Lagae L, Sullivan J, Knupp K, et al. Fenfluramine hydrochloride for the treatment of seizures in Dravet syndrome: a randomised, double‐blind, placebo‐controlled trial. Lancet. 2019;394:2243‐2254. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

