[Effects of non-muscle myosin Ⅱ silenced bone marrow-derived mesenchymal stem cells transplantation on lung extracellular matrix in rats after endotoxin/lipopolysaccharide-induced acute lung injury]
- PMID: 35599418
- PMCID: PMC11705251
- DOI: 10.3760/cma.j.cn501225-20220212-00024
[Effects of non-muscle myosin Ⅱ silenced bone marrow-derived mesenchymal stem cells transplantation on lung extracellular matrix in rats after endotoxin/lipopolysaccharide-induced acute lung injury]
Abstract
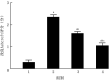
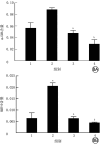
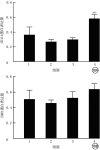
Objective: To investigate the effects of non-muscle myosin Ⅱ (NMⅡ) gene silenced bone marrow-derived mesenchymal stem cells (BMMSCs) on pulmonary extracellular matrix (ECM) and fibrosis in rats with acute lung injury (ALI) induced by endotoxin/lipopolysaccharide (LPS). Methods: The experimental research methods were adopted. Cells from femur and tibial bone marrow cavity of four one-week-old male Sprague-Dawley rats were identified as BMMSCs by flow cytometry, and the third passage of BMMSCs were used in the following experiments. The cells were divided into NMⅡ silenced group transfected with pHBLV-U6-ZsGreen-Puro plasmid containing small interference RNA sequence of NMⅡ gene, vector group transfected with empty plasmid, and blank control group without any treatment, and the protein expression of NMⅡ at 72 h after intervention was detected by Western blotting (n=3). The morphology of cells was observed by an inverted phase contrast microscope and cells labeled with chloromethylbenzoine (CM-DiⅠ) in vitro were observed by an inverted fluorescence microscope. Twenty 4-week-old male Sprague-Dawley rats were divided into blank control group, ALI alone group, ALI+BMMSC group, and ALI+NMⅡ silenced BMMSC group according to the random number table, with 5 rats in each group. Rats in blank control group were not treated, and rats in the other 3 groups were given LPS to induce ALI. Immediately after modeling, rats in ALI alone group were injected with 1 mL normal saline via tail vein, rats in ALI+BMMSC group and ALI+NMⅡ silenced BMMSC group were injected with 1×107/mL BMMSCs and NMⅡ gene silenced BMMSCs of 1 mL labelled with CM-DiⅠ via tail vein, and rats in blank control group were injected with 1 mL normal saline via tail vein at the same time point, respectively. At 24 h after intervention, the lung tissue was collected to observe intrapulmonary homing of the BMMSCs by an inverted fluorescence microscope. Lung tissue was collected at 24 h, in 1 week, and in 2 weeks after intervention to observe pulmonary inflammation by hematoxylin eosin staining and to observe pulmonary fibrosis by Masson staining, and the pulmonary fibrosis in 2 weeks after intervention was scored by modified Ashcroft score (n=5). The content of α-smooth muscle actin (α-SMA), matrix metalloproteinase 2 (MMP-2), and MMP-9 was detected by immunohistochemistry in 2 weeks after intervention (n=3), the activity of superoxide dismutase (SOD), malondialdehyde, myeloperoxidase (MPO) was detected by enzyme-linked immunosorbent assay at 24 h after intervention (n=3), and the protein expressions of CD11b and epidermal growth factor like module containing mucin like hormone receptor 1 (EMR1) in 1 week after intervention were detected by immunofluorescence staining (n=3). Data were statistically analyzed with one-way analysis of variance, Bonferroni method, and Kruskal-Wallis H test. Results: At 72 h after intervention, the NMⅡprotein expression of cells in NMⅡ silenced group was significantly lower than those in blank control group and vector group (with P values <0.01). BMMSCs were in long spindle shape and grew in cluster shaped like vortexes, which were labelled with CM-DiⅠ successfully in vitro. At 24 h after intervention, cell homing in lung of rats in ALI+NMⅡ silenced BMMSC group was more pronounced than that in ALI+BMMSC group, while no CM-DiⅠ-labelled BMMSCs were observed in lung of rats in blank control group and ALI alone group. There was no obvious inflammatory cell infiltration in lung tissue of rats in blank control group at all time points, while inflammatory cell infiltration in lung tissue of rats in ALI+BMMSC group and ALI+NMⅡ silenced BMMSC group was significantly less than that in ALI alone group at 24 h after intervention, and alveolar wall turned to be thinner and a small amount of congestion in local lung tissue appeared in rats of the two groups in 1 week and 2 weeks after intervention. In 1 week and 2 weeks after intervention, collagen fiber deposition in lung tissue of rats in ALI alone group, ALI+BMMSC group, and ALI+NMⅡ silenced BMMSC group was significantly aggravated compared with that in blank control group, while collagen fiber deposition in lung tissue of rats in ALI+BMMSC group and ALI+NMⅡ silenced BMMSC group was significantly improved compared with that in ALI alone group. In 2 weeks after intervention, modified Ashcroft scores for pulmonary fibrosis of rats in ALI alone group, ALI+BMMSC group, and ALI+NMⅡ silenced BMMSC group were 2.36±0.22, 1.62±0.16, 1.06±0.26, respectively, significantly higher than 0.30±0.21 in blank control group (P<0.01). Modified Ashcroft scores for pulmonary fibrosis of rats in ALI+BMMSC group and ALI+NMⅡ silenced BMMSC group were significantly lower than that in ALI alone group (P<0.01), and modified Ashcroft score for pulmonary fibrosis of rats in ALI+NMⅡ silenced BMMSC group was significantly lower than that in ALI+BMMSC group (P<0.01). In 2 weeks after intervention, the content of α-SMA in lung tissue of rats in ALI+BMMSC group and ALI+NMⅡ silenced BMMSC group were significantly decreased compared with that in ALI alone group (P<0.05 or P<0.01). The content of MMP-2 in lung tissue of rats in the 4 groups was similar (P>0.05). The content of MMP-9 in lung tissue of rats in ALI alone group was significantly increased compared with that in blank control group (P<0.01), and the content of MMP-9 in lung tissue of rats in ALI+BMMSC group and ALI+NMⅡ silenced BMMSC group was significantly decreased compared with that in ALI alone group (P<0.01). At 24 h after intervention, the activity of malondialdehyde, SOD, and MPO in lung tissue of rats in ALI alone group, ALI+BMMSC group, and ALI+NMⅡ silenced BMMSC group were significantly increased compared with that in blank control group (P<0.01), the activity of malondialdehyde in lung tissue of rats in ALI+NMⅡ silenced BMMSC group and the activity of SOD in lung tissue of rats in ALI+BMMSC group and ALI+NMⅡ silenced BMMSC group were significantly increased compared with that in ALI alone group (P<0.05 or P<0.01), and the activity of SOD in lung tissue of rats in ALI+NMⅡ silenced BMMSC group was significantly decreased compared with that in ALI+BMMSC group (P<0.01). The activity of MPO in lung tissue of rats in ALI+BMMSC group and ALI+NMⅡ silenced BMMSC group was significantly decreased compared with that in ALI alone group (P<0.01), and the activity of MPO in lung tissue of rats in ALI+NMⅡ silenced BMMSC group was significantly decreased compared with that in ALI+BMMSC group (P<0.01). In 1 week after intervention, the protein expression of CD11b in lung tissue of rats in ALI+NMⅡ silenced BMMSC group was significantly increased compared with those in the other three groups (P<0.05 or P<0.01), while the protein expressions of EMR1 in lung tissue of rats in the four groups were similar (P>0.05). Conclusions: Transplantation of NMⅡ gene silenced BMMSCs can significantly improve the activity of ECM components in the lung tissue in LPS-induced ALI rats, remodel its integrity, and enhance its antioxidant capacity, and alleviate lung injury and pulmonary fibrosis.
目的: 探讨沉默非肌肉肌球蛋白Ⅱ(NMⅡ)基因的骨髓间充质干细胞(BMMSC)对内毒素/脂多糖(LPS)诱导的急性肺损伤(ALI)大鼠肺细胞外基质(ECM)和肺纤维化的影响。 方法: 采用实验研究方法。取4只1周龄雄性SD大鼠股骨和胫骨骨髓腔细胞并经流式细胞术鉴定为BMMSC。取第3代BMMSC用于后续实验。取细胞,分为用含NMⅡ基因的小干扰RNA序列的pHBLV-U6-ZsGreen-Puro质粒转染的沉默NMⅡ组,转染空载质粒的空载组及未进行任何处理的空白对照组,采用蛋白质印迹法检测干预后72 h的NMⅡ的蛋白表达(样本数为3)。取细胞,于倒置相差显微镜下观察形态;另取细胞,用氯甲基苯甲酰胺(CM-DiⅠ)标记,于倒置荧光显微镜下观察体外细胞标记情况。取20只4周龄雄性SD大鼠,按随机数字表法分为空白对照组、单纯ALI组、ALI+BMMSC组及ALI+沉默NMⅡ的BMMSC组,每组5只大鼠。对空白对照组不进行任何处理,其余3组大鼠均给予LPS诱导ALI。造模后即刻,单纯ALI组大鼠经尾静脉注射1 mL生理盐水,ALI+BMMSC组、ALI+沉默NMⅡ的BMMSC组大鼠分别经尾静脉注入1 mL 1×107个/mL CM-DiⅠ标记的BMMSC、NMⅡ基因沉默的BMMSC,空白对照组大鼠于相同时间点通过尾静脉注射1 mL生理盐水。干预后24 h,取肺组织于倒置荧光显微镜下观察BMMSC肺内归巢情况。干预后24 h、1周、2周,取肺组织行苏木精-伊红染色观察肺部炎症情况,行Masson染色观察肺纤维化程度并采用改良Ashcroft评分法对干预后2周肺纤维化程度进行评分(样本数为5)。采用免疫组织化学法检测干预后2周α平滑肌肌动蛋白(α-SMA)、基质金属蛋白酶2(MMP-2)、MMP-9的含量(样本数均为3),采用酶联免疫吸附测定法检测干预后24 h超氧化物歧化酶(SOD)、丙二醛、髓过氧化物酶(MPO)活性(样本数均为3),采用免疫荧光染色法检测干预后1周CD11b和含表皮生长因子样模块黏蛋白样激素受体1(EMR1)蛋白表达(样本数均为3)。对数据行单因素方差分析、Bonferroni法及Kruskal-Wallis H检验。 结果: 干预后72 h,沉默NMⅡ组细胞NMⅡ蛋白表达水平显著低于空白对照组和空载组(P值均<0.01)。BMMSC呈长梭形,簇状生长形如旋涡;CM-DiⅠ体外成功标记BMMSC。干预后24 h,ALI+沉默NMⅡ的BMMSC组大鼠肺内细胞归巢现象较ALI+BMMSC组更明显,而空白对照组和单纯ALI组大鼠肺内未见CM-DiⅠ标记的细胞。空白对照组大鼠干预后各时间点肺组织中均未见明显炎症细胞浸润,ALI+BMMSC组和ALI+沉默NMⅡ的BMMSC组大鼠干预后24 h肺组织炎症细胞浸润较单纯ALI组明显减少,且2组大鼠干预后1、2周肺泡壁变薄,局部肺组织有少量淤血。干预后1、2周,单纯ALI组、ALI+BMMSC组和ALI+沉默NMⅡ的BMMSC组大鼠肺组织胶原纤维沉积均较空白对照组明显加重,但ALI+BMMSC组和ALI+沉默NMⅡ的BMMSC组大鼠肺组织胶原纤维沉积均较单纯ALI组显著改善。干预后2周,单纯ALI组、ALI+BMMSC组和ALI+沉默NMⅡ的BMMSC组大鼠肺纤维化改良Ashcroft评分分别为(2.36±0.22)、(1.62±0.16)、(1.06±0.26)分,均明显高于空白对照组的(0.30±0.21)分(P<0.01),ALI+BMMSC组和ALI+沉默NMⅡ的BMMSC组大鼠肺纤维化改良Ashcroft评分均明显低于单纯ALI组(P<0.01),ALI+沉默NMⅡ的BMMSC组大鼠肺纤维化改良Ashcroft评分明显低于ALI+BMMSC组(P<0.01)。干预后2周,与单纯ALI组比较,ALI+BMMSC组、ALI+沉默NMⅡ的BMMSC组大鼠肺组织α-SMA含量均显著下降(P<0.05或P<0.01)。4组大鼠肺组织MMP-2含量均相近(P>0.05)。与空白对照组比较,单纯ALI组大鼠肺组织MMP-9含量显著升高(P<0.01);与单纯ALI组比较,ALI+BMMSC组、ALI+沉默NMⅡ的BMMSC组大鼠肺组织MMP-9含量均显著降低(P<0.01)。干预后24 h,与空白对照组比较,单纯ALI组、ALI+BMMSC组、ALI+沉默NMⅡ的BMMSC组大鼠肺组织丙二醛、SOD、MPO活性均显著升高(P<0.01);与单纯ALI组比较,ALI+沉默NMⅡ的BMMSC组大鼠肺组织丙二醛活性显著升高(P<0.05),ALI+BMMSC组、ALI+沉默NMⅡ的BMMSC组大鼠肺组织SOD活性均显著升高(P<0.01);与ALI+BMMSC组比较,ALI+沉默NMⅡ的BMMSC组大鼠肺组织SOD活性显著降低(P<0.01)。与单纯ALI组比较,ALI+BMMSC组、ALI+沉默NMⅡ的BMMSC组大鼠肺组织MPO活性均显著降低(P<0.01);与ALI+BMMSC组比较,ALI+沉默NMⅡ的BMMSC组大鼠肺组织MPO活性显著降低(P<0.01)。干预后1周,与其他3组比较,ALI+沉默NMⅡ的BMMSC组大鼠肺组织CD11b蛋白表达显著增加(P<0.05或P<0.01);4组大鼠肺组织EMR1蛋白表达均相近(P>0.05)。 结论: 移植沉默NMⅡ基因的BMMSC能更明显改善LPS所致ALI大鼠肺组织ECM成分的活性,重塑其完整性,增强其抗氧化能力,减轻肺损伤和肺纤维化。.
Conflict of interest statement
Figures







Similar articles
-
[Effects of exosomes from human adipose-derived mesenchymal stem cells on pulmonary vascular endothelial cells injury in septic mice and its mechanism].Zhonghua Shao Shang Yu Chuang Mian Xiu Fu Za Zhi. 2022 Mar 20;38(3):266-275. doi: 10.3760/cma.j.cn501120-20211020-00362. Zhonghua Shao Shang Yu Chuang Mian Xiu Fu Za Zhi. 2022. PMID: 35325972 Free PMC article. Chinese.
-
[Effects and mechanism of annexin A1-overexpressing human adipose-derived mesenchymal stem cells in the treatment of mice with acute respiratory distress syndrome].Zhonghua Shao Shang Yu Chuang Mian Xiu Fu Za Zhi. 2023 May 20;39(5):456-464. doi: 10.3760/cma.j.cn501225-20220408-00130. Zhonghua Shao Shang Yu Chuang Mian Xiu Fu Za Zhi. 2023. PMID: 37805755 Free PMC article. Chinese.
-
[Protective effect of heat shock transcription factor 1 on acute lung injury in septic rats by regulating NOD-like receptor protein 3 inflammasome activation].Zhonghua Wei Zhong Bing Ji Jiu Yi Xue. 2022 Nov;34(11):1167-1172. doi: 10.3760/cma.j.cn121430-20210930-01417. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue. 2022. PMID: 36567560 Chinese.
-
[Intervention of modified Shenling Baizhu San on peritoneal fibrosis induced by peritoneal dialysate with different sugar concentration in rats].Zhonghua Wei Zhong Bing Ji Jiu Yi Xue. 2023 Aug;35(8):875-880. doi: 10.3760/cma.j.cn121430-20220124-00092. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue. 2023. PMID: 37593870 Chinese.
-
[Digoxin alleviates pulmonary fibrosis by regulating phosphatidylinositol-3-kinase/Akt signaling through inhibiting the activation of fibroblast: an in vivo and in vitro experiment].Zhonghua Wei Zhong Bing Ji Jiu Yi Xue. 2022 Nov;34(11):1161-1166. doi: 10.3760/cma.j.cn121430-20220628-00508. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue. 2022. PMID: 36567559 Chinese.
References
-
- 刘 名倬, 王 俊杰, 付 忠华, et al. 沉默非肌肉肌球蛋白ⅡA的骨髓间充质干细胞对烟雾吸入性损伤大鼠早期肺损伤的影响. 中华烧伤杂志. 2017;33(12):766–771. doi: 10.3760/cma.j.issn.1009-2587.2017.12.009. - DOI
-
- 刘 新民. 急性肺损伤与细胞外基质. 国外医学(呼吸系统分册) 1994;14(1):27–29.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
