MiR-19a suppresses ferroptosis of colorectal cancer cells by targeting IREB2
- PMID: 35599631
- PMCID: PMC9275930
- DOI: 10.1080/21655979.2022.2054194
MiR-19a suppresses ferroptosis of colorectal cancer cells by targeting IREB2
Abstract
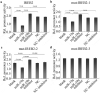
Colorectal cancer (CRC) is the most common malignant tumor occurred in digestive system. However, the prognosis of CRC patients is poor. Therefore, it is urgent to illuminate the mechanism suppressing CRC and explore novel targets or therapies for CRC treatment. MicroRNAs (miRNAs) are a class of non-coding RNAs with a length of 20-23 nucleotides encoded by endogenous genes, which are associated with the development of a variety of cancers, including CRC. Studies have shown that miR-19a is identified as oncogenic miRNA and promotes the proliferation, migration and invasion of CRC cells. However, the relationship between miR-19a and ferroptosis in CRC remains unknown. Here, we reported that iron-responsive element-binding protein 2 (IREB2), as an inducer of ferroptosis, was negatively regulated by miR-19a. IREB2 is a direct target of miR-19a. In addition, ferroptosis was suppressed by miR-19a through inhibiting IREB2. Thus, we proposed a novel mechanism of ferroptosis mediated by miR-19a in CRC cells, which could give rise to a new strategy for the therapy of CRC.
Keywords: cell proliferation; colorectal cancer; ferroptosis; iron-responsive element-binding protein 2; miR-19a; miRNA.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures



Similar articles
-
miR-19a promotes colorectal cancer proliferation and migration by targeting TIA1.Mol Cancer. 2017 Mar 4;16(1):53. doi: 10.1186/s12943-017-0625-8. Mol Cancer. 2017. PMID: 28257633 Free PMC article.
-
MiR-19a enhances cell proliferation, migration, and invasiveness through enhancing lymphangiogenesis by targeting thrombospondin-1 in colorectal cancer.Biochem Cell Biol. 2019 Dec;97(6):731-739. doi: 10.1139/bcb-2018-0302. Epub 2019 Jun 14. Biochem Cell Biol. 2019. PMID: 31199884
-
miR-29a-3p in Exosomes from Heme Oxygenase-1 Modified Bone Marrow Mesenchymal Stem Cells Alleviates Steatotic Liver Ischemia-Reperfusion Injury in Rats by Suppressing Ferroptosis via Iron Responsive Element Binding Protein 2.Oxid Med Cell Longev. 2022 Jun 9;2022:6520789. doi: 10.1155/2022/6520789. eCollection 2022. Oxid Med Cell Longev. 2022. PMID: 35720183 Free PMC article.
-
Decoding the interaction between miR-19a and CBX7 focusing on the implications for tumor suppression in cancer therapy.Med Oncol. 2023 Dec 19;41(1):21. doi: 10.1007/s12032-023-02251-y. Med Oncol. 2023. PMID: 38112798 Review.
-
Ferroptosis in colorectal cancer: Potential mechanisms and effective therapeutic targets.Biomed Pharmacother. 2022 Sep;153:113524. doi: 10.1016/j.biopha.2022.113524. Epub 2022 Aug 13. Biomed Pharmacother. 2022. PMID: 36076606 Review.
Cited by
-
The Regulation of Ferroptosis by Noncoding RNAs.Int J Mol Sci. 2023 Aug 28;24(17):13336. doi: 10.3390/ijms241713336. Int J Mol Sci. 2023. PMID: 37686142 Free PMC article. Review.
-
Inhibition of miR-9-3p facilitates ferroptosis by activating SAT1/p53 pathway in lung adenocarcinoma.Transl Lung Cancer Res. 2024 Dec 31;13(12):3426-3442. doi: 10.21037/tlcr-24-762. Epub 2024 Dec 27. Transl Lung Cancer Res. 2024. PMID: 39830759 Free PMC article.
-
MicroRNAs at the crossroads of exercise and ferroptosis: a regulatory bridge.Clin Exp Med. 2025 Jul 6;25(1):234. doi: 10.1007/s10238-025-01766-0. Clin Exp Med. 2025. PMID: 40618320 Free PMC article. Review.
-
Ferroptosis as a key player in the pathogenesis and intervention therapy in liver injury: focusing on drug-induced hepatotoxicity.Naunyn Schmiedebergs Arch Pharmacol. 2025 Apr 17. doi: 10.1007/s00210-025-04115-w. Online ahead of print. Naunyn Schmiedebergs Arch Pharmacol. 2025. PMID: 40244448 Review.
-
Therapeutic efficacy of ferroptosis in the treatment of colorectal cancer (Review).Oncol Lett. 2024 Sep 26;28(6):563. doi: 10.3892/ol.2024.14697. eCollection 2024 Dec. Oncol Lett. 2024. PMID: 39390976 Free PMC article. Review.
References
-
- Wrobel P, Ahmed S.. Current status of immunotherapy in metastatic colorectal cancer. Int J Colorectal Dis. 2019;34(1):13–25. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
