Sexual Dimorphism in the Effect of Neonatal Inflammatory Pain on Stress Reactivity of Hormonal Response and Cognitive Functions in Adult Rats
- PMID: 35599637
- PMCID: PMC9109674
- DOI: 10.1134/S0022093022020053
Sexual Dimorphism in the Effect of Neonatal Inflammatory Pain on Stress Reactivity of Hormonal Response and Cognitive Functions in Adult Rats
Abstract
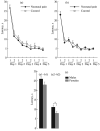
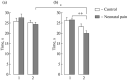
The effect of moderate neonatal stress induced by inflammatory pain in rat pups of both sexes on the hormonal response and cognitive processes in adult animals was studied in the Morris water maze. No significant differences in spatial learning and memory were found in experimental rats exposed to neonatal inflammatory pain vs. control animals. However, experimental rats exhibited sex differences in long-term spatial memory whose efficiency was higher in males vs. females. After long-term memory testing, stress responsiveness of the hypothalamic-pituitary-adrenocortical axis, as assessed by the plasma corticosterone level in the formalin test, was higher in experimental males vs. females. Only experimental females exhibited differences between short-term and long-term memory, with the efficiency being higher in the former. Thus, sexual dimorphism was found in the effect of neonatal nociceptive stress on long-term spatial memory in adult rats: experimental males vs. females demonstrated more effective long-term memory combined with a higher stress reactivity of the hormonal response.
Keywords: adult rats; corticosterone; neonatal inflammatory pain; spatial learning and memory.
© Pleiades Publishing, Ltd. 2022.
Conflict of interest statement
CONFLICT OF INTERESTThe authors declare that they have neither evident nor potential conflict of interest related to the publication of this article.
Figures
Similar articles
-
The Long-Term Effects of Neonatal Inflammatory Pain on Cognitive Function and Stress Hormones Depend on the Heterogeneity of the Adolescent Period of Development in Male and Female Rats.Front Behav Neurosci. 2021 Jul 21;15:691578. doi: 10.3389/fnbeh.2021.691578. eCollection 2021. Front Behav Neurosci. 2021. PMID: 34366805 Free PMC article.
-
Long-Term Effect of Moderate Hypoxia and Chronic Administration of Fluoxetine during the Neonatal Period on Cognitive and Stress-Hormonal Functions in Adult Male Rats.Bull Exp Biol Med. 2023 Jun;175(2):196-200. doi: 10.1007/s10517-023-05853-8. Epub 2023 Jul 20. Bull Exp Biol Med. 2023. PMID: 37470895
-
[DIFFERENCES IN ADAPTIVE BEHAVIORS IN ADOLESCENT MALE AND FEMALE RATS EXPOSED AS NEWBORNS TO INFLAMMATORY PAIN OR STRESS].Zh Evol Biokhim Fiziol. 2015 Jul-Aug;51(4):266-75. Zh Evol Biokhim Fiziol. 2015. PMID: 26547951 Russian.
-
Environmental and tactile stimulation modulates the neonatal handling effect on adult rat spatial memory.Int J Dev Neurosci. 2009 Dec;27(8):747-55. doi: 10.1016/j.ijdevneu.2009.08.013. Epub 2009 Aug 29. Int J Dev Neurosci. 2009. PMID: 19720127
-
Sex differences in chronic stress effects on memory in rats.Stress. 2002 Sep;5(3):205-16. doi: 10.1080/1025389021000010549. Stress. 2002. PMID: 12186683 Review.
References
-
- De Kort AR, Joosten EAJ, Patijn J, Tibboel D, van den Hoogen NJ (2021) The development of descending serotonergic modulation of the spinal nociceptive network: a life span perspective. Pediatr Res Online ahead of print. 10.1038/s41390-021-01638-9 - PubMed
LinkOut - more resources
Full Text Sources
